Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00326
|
|||||
| Drug Name |
Sarcosine
|
|||||
| Synonyms |
(Methylamino)acetic acid; (Methylamino)ethanoic acid; 2-(methylamino)acetic acid; AI3-15410; Acetic acid, (methylamino)-; BRN 1699442; CHEBI:15611; CHEMBL304383; Cocoylsarcosine; EINECS 203-538-6; FSYKKLYZXJSNPZ-UHFFFAOYSA-N; Glycine, N-methyl-; H-Sar-OH; Methylamino-Acetic Acid; Methylaminoacetic acid; Methylglycine; N-Methyl glycine; N-Methylaminoacetic acid; N-methyl-Glycine; N-methylglycine; Polysarcosine; SAR; Sarcosin; Sarcosine, 98%; Sarcosinic acid; Sargosine hydrochloride; UNII-Z711V88R5F; Z711V88R5F
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Schizophrenia [ICD11: 6A20] | Phase 2 | [1] | |||
| Structure |
|
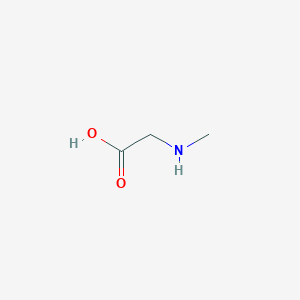 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C3H7NO2
|
|||||
| Canonical SMILES |
CNCC(=O)O
|
|||||
| InChI |
InChI=1S/C3H7NO2/c1-4-2-3(5)6/h4H,2H2,1H3,(H,5,6)
|
|||||
| InChIKey |
FSYKKLYZXJSNPZ-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 107-97-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 89.09 | Topological Polar Surface Area | 49.3 | ||
| Heavy Atom Count | 6 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
-2.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
102851555
, 103260070
, 103928493
, 104296726
, 104668562
, 10503566
, 117480502
, 123055190
, 124799851
, 125270146
, 125324385
, 125337494
, 126523128
, 126595739
, 126604360
, 126646789
, 126676630
, 127319579
, 127319580
, 14842807
, 24439439
, 24848035
, 24899761
, 3135884
, 3513
, 38327572
, 413393
, 50045496
, 50370559
, 5077689
, 51075215
, 57260822
, 57264366
, 57304408
, 57320726
, 589772
, 608062
, 7888914
, 7890376
, 7979965
, 8143301
, 8150965
, 85089252
, 85164986
, 85244545
, 87243947
, 87350276
, 87572403
, 88828665
, 92297466
|
|||||
| ChEBI ID |
CHEBI:15611
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | GLYT1 | Transporter Info | Sodium- and chloride-dependent glycine transporter 1 | Substrate | [2] | |
| References | ||||||
| 1 | ClinicalTrials.gov (NCT01503359) Effect of Sarcosine on Symptomatology, Quality of Life, Oxidative Stress and Glutamatergic Parameters in Schizophrenia | |||||
| 2 | Glycine is taken up through GLYT1 and GLYT2 transporters into mouse spinal cord axon terminals and causes vesicular and carrier-mediated release of its proposed co-transmitter GABA. J Neurochem. 2001 Mar;76(6):1823-32. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
