Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00331
|
|||||
| Drug Name |
Sumatriptan
|
|||||
| Synonyms |
(3-[2-(Dimethylamino)ethyl]-1H-indol-5-yl)-N-methylmethanesulfonamide; 1-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]-N-methyl-methanesulfonamide; 1-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]-N-methylmethanesulfonamide; 1-{3-[2-(dimethylamino)ethyl]-1H-indol-5-yl}-N-methylmethanesulfonamide; 3-(2-(Dimethylamino)ethyl)-N-methyl-1H-indole-5-methanesulfonamide; 3-[2-(Dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulfonamide; 3-[2-(Dimethylamino)ethyl]-N-methylindole-5-methanesulfonamide; GR 43175; GR 43175X; GR-43175; Imigran; Imigran (TN); Imitrex; Imitrex (TN); KS-1116; NP101; Sumatran; Sumatriptan (JAN/USP/INN); Sumatriptanum; Sumatriptanum [INN-Latin]; Sumax
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Migraine Headaches [ICD11: 8A80] | Approved | [1] | |||
| Therapeutic Class |
Vasoconstrictor Agents
|
|||||
| Structure |
|
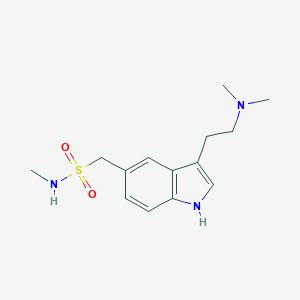 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C14H21N3O2S
|
|||||
| Canonical SMILES |
CNS(=O)(=O)CC1=CC2=C(C=C1)NC=C2CCN(C)C
|
|||||
| InChI |
InChI=1S/C14H21N3O2S/c1-15-20(18,19)10-11-4-5-14-13(8-11)12(9-16-14)6-7-17(2)3/h4-5,8-9,15-16H,6-7,10H2,1-3H3
|
|||||
| InChIKey |
KQKPFRSPSRPDEB-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 103628-46-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 295.4 | Topological Polar Surface Area | 73.6 | ||
| Heavy Atom Count | 20 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
0.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103188807
, 103919260
, 104309008
, 10502764
, 119526324
, 124799677
, 125336887
, 126525330
, 126667002
, 128419875
, 134337534
, 135018088
, 135651095
, 137002439
, 142467611
, 144205081
, 14718471
, 14825017
, 152034343
, 160964014
, 162182413
, 164814822
, 165699400
, 170465377
, 172866507
, 174006309
, 175265694
, 25819952
, 26612933
, 26749855
, 29224410
, 46506520
, 48334598
, 48416587
, 49679316
, 49984205
, 50107736
, 50422744
, 53790264
, 56322605
, 5634131
, 57322735
, 7847517
, 7980713
, 81040912
, 8153293
, 85209322
, 85789242
, 93166406
, 9527
|
|||||
| ChEBI ID |
ChEBI:10650
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Sumatriptan was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42. | |||||
| 3 | HT29-MTX and Caco-2/TC7 monolayers as predictive models for human intestinal absorption: role of the mucus layer. J Pharm Sci. 2001 Oct;90(10):1608-19. Comparative Study | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
