Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00382
|
|||||
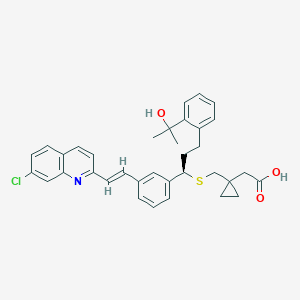
| Drug Name |
Montelukast
|
|||||
| Synonyms |
(R-(E))-1-(((1-(3-(2-(7-Chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)thio)methyl)cyclopropaneacetic acid; 1-((((1R)-1-(3-((1E)-2-(7-Chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)thio)methyl)cyclopropaneacetic acid; 2-[1-[[(1R)-1-[3-[(E)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(2-hydroxypropan-2-yl)phenyl]propyl]sulfanylmethyl]cyclopropyl]acetic acid; Apxi toxin; Brondilat; Brondilat (TN); MK 0476; MK-0476; Montair; Montelukast (INN); Montelukast [INN:BAN]; Singulair (TN); Singular; Sodium 1-(((1-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)thio)methyl)cyclopropylacetate; {1-[({(1R)-1-{3-[(E)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl}-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl}sulfanyl)methyl]cyclopropyl}acetic acid
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Asthma [ICD11: CA23] | Approved | [1] | |||
| Therapeutic Class |
Antiasthmatic Agents
|
|||||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C35H36ClNO3S
|
|||||
| Canonical SMILES |
CC(C)(C1=CC=CC=C1CCC(C2=CC=CC(=C2)C=CC3=NC4=C(C=CC(=C4)Cl)C=C3)SCC5(CC5)CC(=O)O)O
|
|||||
| InChI |
InChI=1S/C35H36ClNO3S/c1-34(2,40)30-9-4-3-7-25(30)13-17-32(41-23-35(18-19-35)22-33(38)39)27-8-5-6-24(20-27)10-15-29-16-12-26-11-14-28(36)21-31(26)37-29/h3-12,14-16,20-21,32,40H,13,17-19,22-23H2,1-2H3,(H,38,39)/b15-10+/t32-/m1/s1
|
|||||
| InChIKey |
UCHDWCPVSPXUMX-TZIWLTJVSA-N
|
|||||
| CAS Number |
CAS 158966-92-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 586.2 | Topological Polar Surface Area | 95.7 | ||
| Heavy Atom Count | 41 | Rotatable Bond Count | 12 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
7.7
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103207442
, 103948950
, 113854447
, 124899385
, 126681707
, 127278617
, 127278618
, 127278619
, 127278620
, 127278621
, 127278622
, 127278623
, 127278624
, 127278625
, 127278626
, 127278627
, 127278628
, 127278629
, 127278630
, 127278631
, 127278632
, 127278633
, 127278634
, 127278635
, 127278636
, 134337621
, 135039402
, 137156298
, 140071379
, 14886895
, 14886896
, 160963817
, 162011440
, 163669670
, 172440032
, 175268932
, 175612182
, 39289972
, 46505585
, 48416293
, 50064526
, 53786760
, 56352853
, 57357981
, 75937172
, 8616482
, 92308743
, 93166552
, 96024917
, 9685
|
|||||
| ChEBI ID |
ChEBI:50730
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [2] | |
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [3] | ||
| References | ||||||
| 1 | Montelukast was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Effect of citrus juice and SLCO2B1 genotype on the pharmacokinetics of montelukast. J Clin Pharmacol. 2011 May;51(5):751-60. | |||||
| 3 | Effects of polymorphisms of the SLCO2B1 transporter gene on the pharmacokinetics of montelukast in humans. J Clin Pharmacol. 2013 Nov;53(11):1186-93. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
