Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00407
|
|||||
| Drug Name |
Nateglinide
|
|||||
| Synonyms |
(2R)-3-phenyl-2-[(4-propan-2-ylcyclohexanecarbonyl)amino]propanoic acid; 3-phenyl-2-[(4-propan-2-ylcyclohexanecarbonyl)amino]propanoic acid; A-4166; AY-4166; DJN 608; DJN-608; Fastic; IPCCPA; N-((4-isopropylcyclohexyl)carbonyl)phenylalanine; N-{[4-(propan-2-yl)cyclohexyl]carbonyl}phenylalanine; N-{[trans-4-(propan-2-yl)cyclohexyl]carbonyl}-D-phenylalanine; Nate-glinide; Nateglinide [INN]; Nateglinide, (D-Phe)-isomer; Nateglinide, (cis,D-Phe)-isomer; Novartis brand of nateglinide; SDZ-DJN-608; Senaglinide; Starlix; Starlix (TN); Starsis; Trazec; YM-026
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Diabetes [ICD11: 5A10-5A14] | Approved | [1] | |||
| Therapeutic Class |
Hypoglycemic Agents
|
|||||
| Structure |
|
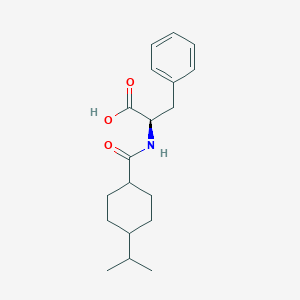 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C19H27NO3
|
|||||
| Canonical SMILES |
CC(C)C1CCC(CC1)C(=O)NC(CC2=CC=CC=C2)C(=O)O
|
|||||
| InChI |
InChI=1S/C19H27NO3/c1-13(2)15-8-10-16(11-9-15)18(21)20-17(19(22)23)12-14-6-4-3-5-7-14/h3-7,13,15-17H,8-12H2,1-2H3,(H,20,21)(H,22,23)/t15?,16?,17-/m1/s1
|
|||||
| InChIKey |
OELFLUMRDSZNSF-OFLPRAFFSA-N
|
|||||
| CAS Number |
CAS 105816-04-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 317.4 | Topological Polar Surface Area | 66.4 | ||
| Heavy Atom Count | 23 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
3.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103206766
, 104253649
, 114155796
, 117890894
, 12013966
, 124658899
, 124757828
, 124801358
, 125164632
, 126630653
, 126657177
, 128797137
, 128797139
, 128797141
, 131293719
, 131347903
, 131860350
, 135692483
, 136949105
, 137129256
, 137175459
, 137231395
, 140330680
, 142491149
, 142619974
, 143248460
, 144204985
, 14752539
, 14874578
, 151981031
, 152100318
, 26719880
, 37101837
, 39341005
, 46386701
, 46504836
, 49681657
, 49830241
, 56313768
, 57359430
, 582898
, 75919327
, 7848174
, 7980094
, 81092826
, 91748929
, 92308370
, 92308686
, 92711649
, 93166415
|
|||||
| ChEBI ID |
ChEBI:31897
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MCT6 | Transporter Info | Monocarboxylate transporter 6 | Substrate | [2] | |
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [3] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [4] | ||
| References | ||||||
| 1 | Nateglinide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Quercetin, Morin, Luteolin, and Phloretin Are Dietary Flavonoid Inhibitors of Monocarboxylate Transporter 6. Mol Pharm. 2017 Sep 5;14(9):2930-2936. | |||||
| 3 | Organic Anion Transporter 2-Mediated Hepatic Uptake Contributes to the Clearance of High-Permeability-Low-Molecular-Weight Acid and Zwitterion Drugs: Evaluation Using 25 Drugs. J Pharmacol Exp Ther. 2018 Nov;367(2):322-334. | |||||
| 4 | FDA Drug Development and Drug Interactions | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
