Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00410
|
|||||
| Drug Name |
Lovastatin
|
|||||
| Synonyms |
2beta,6alpha-Dimethyl-8alpha-(2-methyl-1-oxobutoxy)-mevinic acid lactone; 6 Methylcompactin; 6 alpha-Methylcompactin; 6-Methylcompactin; 6-alpha-Methylcompactin; 6alpha-Methylcompactin; Advicor (TN); Altocor; Altocor (TN); Altoprev; Altoprev (TN); Artein; Belvas; Cholestra; Closterol; Colevix; Hipolip; Hipovastin; L-154803; Lestatin; Lipdip; Lipivas; Lipofren; Liposcler; Lovalip; Lovalord; Lovastatin & Primycin; Lovastatin (USP/INN); Lovastatin [USAN:BAN:INN]; Lovastatin, (1 alpha(S*))-Isomer; Lovastatin, 1 alpha-Isomer (without R*/S* notation); Lovastatina; Lovastatina [Spanish]; Lovastatine; Lovastatine [French]; Lovastatinum; Lovastatinum [Latin]; Lovasterol; Lovastin; Lozutin; MK 803; MK-803; MK803; ML-530B; MSD 803; Mevacor; Mevacor (TN); Mevinacor; Mevinolin; Mevinolin from Aspergillus sp.; Mevlor; Monacolin K; Monakolin K; Nergadan; Paschol; Rextat; Rodatin; Rovacor; Sivlor;Taucor; Statosan (TN); Tecnolip; Teroltrat
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Hypercholesterolemia [ICD11: 5C80.0] | Approved | [1] | |||
| Therapeutic Class |
Anticholesteremic Agents
|
|||||
| Structure |
|
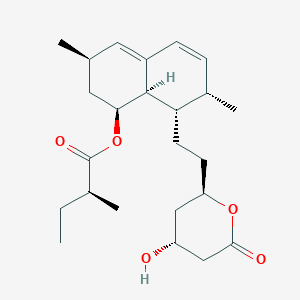 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C24H36O5
|
|||||
| Canonical SMILES |
CCC(C)C(=O)OC1CC(C=C2C1C(C(C=C2)C)CCC3CC(CC(=O)O3)O)C
|
|||||
| InChI |
InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19+,20-,21-,23-/m0/s1
|
|||||
| InChIKey |
PCZOHLXUXFIOCF-BXMDZJJMSA-N
|
|||||
| CAS Number |
CAS 75330-75-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 404.5 | Topological Polar Surface Area | 72.8 | ||
| Heavy Atom Count | 29 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
4.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321765
, 10852021
, 11336207
, 11361446
, 11372945
, 11462418
, 11466544
, 11467664
, 11486128
, 11491688
, 11528634
, 12012664
, 12146073
, 14806053
, 14879393
, 17389841
, 22395934
, 24896706
, 25622285
, 26612569
, 26680612
, 26697338
, 26751557
, 26759065
, 34717349
, 46391668
, 46508223
, 47515402
, 47589078
, 47662390
, 47662391
, 47662392
, 47736583
, 47959858
, 47959859
, 48035229
, 48185079
, 48334593
, 48413948
, 48416187
, 496591
, 623233
, 7734777
, 7847425
, 7885508
, 7979813
, 8150103
, 8182985
, 855905
, 9285
|
|||||
| ChEBI ID |
ChEBI:40303
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Lovastatin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem. 1999 Dec 24;274(52):37161-8. | |||||
| 3 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
