Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00412
|
|||||
| Drug Name |
Efavirenz
|
|||||
| Synonyms |
(-)-Efavirenz; (4S)-6-Chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one; (4S)-6-Chloro-4-cyclopropylethynyl-4-trifluoromethyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one; (4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-1H-3,1-benzoxazin-2-one; (4S)-6-chloro-4-(cyclopropylethynyl)-4-(trifluoromethyl)-1,4-dihydro-2H-3,1-benzoxazin-2-one; (S)-6-Chloro-4-(2-cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-ben; (S)-6-Chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one; (S)-6-Chloro-4-cyclopropylethynyl-4-trifluoromethyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one; 2H-3,1-Benzoxazin-2-one, 6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-, (4S)-(9; 6-chloro-4-(2-cyclopropyl-1-ethynyl)-4-trifluoromethyl-(4S)-1,4-dihydro-2H-benzo[d][1,3]oxazin-2-one; DMP 266; DMP-266; EFV; EFZ; Efavirenz (JAN/INN); Efavirenz, (S)-isomer; Eravirenz; L 743726; L-741211; L-743,726; L-743725; L-743726; Met-SDF-1.beta. & Efavirenz; Met-Stromal Cell-derived Factor-1.beta. (Human) & Efavirenz; Stocrin; Stocrin (TN); Strocin (TM); Sustiva; Sustiva (TM); Sustiva (TN); Zoxazin-2-one
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Human immunodeficiency virus infection [ICD11: 1C62.Z] | Approved | [1] | |||
| Therapeutic Class |
Anti-HIV Agents
|
|||||
| Structure |
|
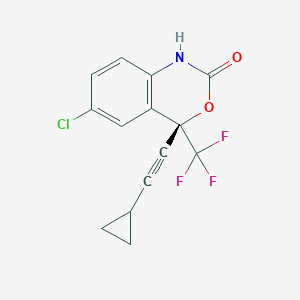 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C14H9ClF3NO2
|
|||||
| Canonical SMILES |
C1CC1C#CC2(C3=C(C=CC(=C3)Cl)NC(=O)O2)C(F)(F)F
|
|||||
| InChI |
InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1
|
|||||
| InChIKey |
XPOQHMRABVBWPR-ZDUSSCGKSA-N
|
|||||
| CAS Number |
CAS 154598-52-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 315.67 | Topological Polar Surface Area | 38.3 | ||
| Heavy Atom Count | 21 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10288
, 104234240
, 104330198
, 118313744
, 12014858
, 124658998
, 126592964
, 126608811
, 126622440
, 126654055
, 129722732
, 131300250
, 134337998
, 135022248
, 136367956
, 136903808
, 137005170
, 137229934
, 14850291
, 2102933
, 26719858
, 43121048
, 46386724
, 46392227
, 46392228
, 46506827
, 49681705
, 50064506
, 53788925
, 57315036
, 615076
, 644086
, 78192089
, 7847960
, 7887287
, 7979148
, 8032270
, 8189153
, 819805
, 819816
, 822777
, 822778
, 823557
, 87350529
, 91146581
, 92308418
, 92309272
, 92717165
, 93166541
, 99444180
|
|||||
| ChEBI ID |
ChEBI:119486
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP3 | Transporter Info | Multidrug resistance-associated protein 3 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Efavirenz was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Induction of multiple drug transporters by efavirenz. J Pharmacol Sci. 2009 Feb;109(2):242-50. | |||||
| 3 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
