Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00469
|
|||||
| Drug Name |
Carboplatin
|
|||||
| Synonyms |
(SP-4-2)-diammine[cyclobutane-1,1-dicarboxylato(2-)-kappa(2)O,O']platinum; 1,1-Cyclobutanedicarboxylate diammine platinum (II); 1,1-Cyclobutanedicarboxylate diammine platinum(II); Azanide; C 2538; Carbopaltin; Carboplatin (JAN/USP/INN); Carboplatin (USAN); Carboplatin [USAN:INN:BAN:JAN]; Carboplatine; Carboplatine [French]; Carboplatino; Carboplatino [Spanish]; Carboplatinum; Carboplatinum [Latin]; Cbdca; Cis-(1,1-Cyclobutanedicarboxylato)diammineplatinum(II); Cis-Diamine(1,1-cyclobutanedicarboxylato)platinum(II); Cis-Diamine[1,1-cyclobutanedicarboxylato]platinum(II); Cis-Diammine(1,1-cyclobutanedicarboxylato) platinum; Cis-Diammine(1,1-cyclobutanedicarboxylato)platinum; Cis-Diammine(1,1-cyclobutanedicarboxylato)platinum(II); Cis-Diammine(cyclobutanedicarboxylato)platinumII; Cis-Diammine[1,1-cyclobutane-dicarboxylato] platinum; Cyclobutane-1,1-dicarboxylate; Cyclobutane-1,1-dicarboxylic acid; Diammine(1,1-cyclobutanedicarboxylato)platinum (II); Diammine(cyclobutane-1,1-dicarboxylato(2-)-O,O')platinum; Diammine-1,1-cyclobutane dicarboxylate platinum II; Diammine[cyclobutane-1,1-dicarboxylato(2-)-k2O1,O1]platinum; Ercar; IUPAC: Azane; JM 8; JM-8; Paraplatin; Paraplatin (TN); Paraplatin, Carboplatin; Paraplatin-AQ; Platinum(+2) Cation; Platinum(2+); Platinum(II), (1, 1-cyclobutanedicar; Platinum, diammine(1,1-cyclobutanedicarboxylato(2-)-O,O')-, (SP-4-2); Platinum, {diammine[1,1-cyclobut
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Ovarian cancer [ICD11: 2C73] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
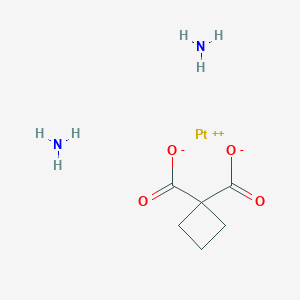
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C6H12N2O4Pt
|
|||||
| Canonical SMILES |
C1CC(C1)(C(=O)[O-])C(=O)[O-].N.N.[Pt+2]
|
|||||
| InChI |
InChI=1S/C6H8O4.2H3N.Pt/c7-4(8)6(5(9)10)2-1-3-6;;;/h1-3H2,(H,7,8)(H,9,10);2*1H3;/q;;;+2/p-2
|
|||||
| InChIKey |
OLESAACUTLOWQZ-UHFFFAOYSA-L
|
|||||
| CAS Number |
CAS 41575-94-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 371.25 | Topological Polar Surface Area | 82.3 | ||
| Heavy Atom Count | 13 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| PubChem CID | ||||||
| PubChem SID |
117682520
, 127293893
, 127293894
, 127293895
, 127293896
, 127293897
, 127293898
, 127293899
, 127293900
, 127293901
, 127293902
, 127293903
, 127293904
, 127293905
, 127293906
, 127293907
, 127293908
, 127293909
, 127293910
, 127293911
, 127293912
, 127293913
, 127293914
, 127293915
, 127293916
, 127293917
, 127293918
, 127293919
, 127293920
, 127293921
, 127293922
, 127293923
, 127293924
, 127293925
, 127293926
, 127293927
, 127293928
, 127293929
, 127293930
, 127293931
, 127293932
, 127293933
, 127293934
, 127293935
, 127293936
, 15350457
, 22704376
, 33972238
, 75354552
, 87558792
|
|||||
| ChEBI ID |
ChEBI:31355
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | CTR1 | Transporter Info | High affinity copper uptake protein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Carboplatin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Overcoming platinum drug resistance with copper-lowering agents. Anticancer Res. 2013 Oct;33(10):4157-61. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
