Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00480
|
|||||
| Drug Name |
Cyclacillin
|
|||||
| Synonyms |
(1-Aminocyclohexyl)penicillin; (2S,5R,6R)-6-[(1-aminocyclohexanecarbonyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-{[(1-aminocyclohexyl)carbonyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(1-aminocyclohexanecarboxamido)-3,3-dimethyl-7-oxo-(8CI); 6-(1-Aminocyclohexanecarboxamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid; 6-(1-Aminocyclohexanecarboxamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 6-(1-Aminocyclohexanecarboxamido)penicillanic acid; 6-(1-Aminocyclohexylcarboxamido)penicillanic acid; 6beta-(1-aminocyclohexanecarboxamido)-2,2-dimethylpenam-3alpha-carboxylic acid; AC 98; Aminocyclohexyl penicillin; Aminocyclohexylpenicillin; Bastcillin; C-12104; Calthor; Ciclacilina; Ciclacilina [INN-Spanish]; Ciclacillin; Ciclacillin (JP15/INN); Ciclacilline; Ciclacilline [INN-French]; Ciclacillinum; Ciclacillinum [INN-Latin]; Ciclacillum; Citosarin; Cyclacillin (USAN); Cyclacillin [USAN]; Cyclapen; Cyclapen (TN); Cyclapen-W; Cyclapen-W (TN); Noblicil; Orfilina; Peamezin; Syngacillin; Ultracillin; Vastcillin; Vastcillin (TN); Vipicil; WY 4508; WY4508; Wy-4508; Wyvital
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Bacterial infections [ICD11: 1A00-1H0Z] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
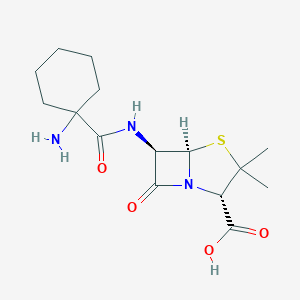
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C15H23N3O4S
|
|||||
| Canonical SMILES |
CC1(C(N2C(S1)C(C2=O)NC(=O)C3(CCCCC3)N)C(=O)O)C
|
|||||
| InChI |
InChI=1S/C15H23N3O4S/c1-14(2)9(12(20)21)18-10(19)8(11(18)23-14)17-13(22)15(16)6-4-3-5-7-15/h8-9,11H,3-7,16H2,1-2H3,(H,17,22)(H,20,21)/t8-,9+,11-/m1/s1
|
|||||
| InChIKey |
HGBLNBBNRORJKI-WCABBAIRSA-N
|
|||||
| CAS Number |
CAS 3485-14-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 341.4 | Topological Polar Surface Area | 138 | ||
| Heavy Atom Count | 23 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
1.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103770339
, 103914475
, 104346698
, 11112536
, 11446317
, 11467148
, 11468268
, 11486856
, 124766183
, 128055795
, 134224408
, 134338404
, 134985468
, 136357160
, 137012596
, 137178974
, 144204085
, 15075673
, 160964336
, 162516292
, 178101519
, 179149343
, 198937842
, 226420447
, 29286625
, 396250
, 46508073
, 47291276
, 47365368
, 47440419
, 48259416
, 48415821
, 49699306
, 49956857
, 50123946
, 57269579
, 57330251
, 583155
, 602914
, 74457515
, 7848397
, 8139302
, 8164183
, 85788579
, 92126086
|
|||||
| ChEBI ID |
ChEBI:31444
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [2] | |
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [2] | ||
| References | ||||||
| 1 | Cyclacillin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Transport characteristics of a novel peptide transporter 1 substrate, antihypotensive drug midodrine, and its amino acid derivatives. J Pharmacol Exp Ther. 2006 Jul;318(1):455-60. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
