Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00494
|
|||||
| Drug Name |
Estrone
|
|||||
| Synonyms |
(13S)-3-hydroxy-13-methyl-7,8,9,11,12,13,15,16-octahydro-6H-cyclopenta[a]phenanthren-17(14H)-one; (8R,9S,13S,14S)-3-hydroxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-one;1,3,5(10)-Estratrien-3-ol-17-one; 1,3,5(10)-Oestratrien-3-ol-17-one; 3-Hydroxy-1,3,5(10)-estratrien-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; 3-Hydroxy-17-keto-oestra-1,3,5-triene; 3-Hydroxy-oestra-1,3,5(10)-trien-17-one; 3-Hydroxyestra-1,3,5(10)-trien-17-one; 3-Hydroxyestra-1,3,5(10)-triene-17-one; 3-hydroxy-estra-1,3,5(10)-trien-17-one; Aquacrine; Crinovaryl; Cristallovar; Crystogen; Delta-1,3,5-Estratrien-3beta-ol-17-one; Delta-1,3,5-Oestratrien-3beta-ol-17-one; Delta-1,3,5-estratrien-3-beta-ol-17-one; Delta-1,3,5-oestratrien-3-beta-ol-17-one; Destrone; Disynformon; E 9750; E(sub 1); E0026; Endofolliculina; Esterone; Estrin; Estrogenic Substance; Estrol; Estron; Estrona; Estrona [INN-Spanish]; Estrona [Spanish]; Estrone (E1); Estrone (JAN/USP/INN); Estrone (TN); Estrone [USAN:INN]; Estrone, (+-)-Isomer; Estrone, (8 alpha)-Isomer; Estrone, (9 beta)-Isomer; Estrone-A; Estronum; Estronum [INN-Latin]; Estrovarin; Estrugenone; Estrusol; Fem-O-Gen; Femestrone inj.; Femestrone injection; Femidyn; Fermidyn; Folikrin; Folipex; Folisan; Follestrine; Follestrol; Follicular hormone; Folliculin; Folliculine; Folliculine benzoate; Follicunodis; Glandubolin; Hauck Brand of Estrone; Hiestrone; Hormestrin; Hormofollin; Hormovarine; Hyrex Brand of Estrone; Kestrone; Ketodestrin; Ketohydroxy-Estratriene; Ketohydroxyestrin; Ketohydroxyoestrin; Ketophydroxyestrin; Kolpon; Menagen; Menformon; Menformon A; Mestronaq; NATURAL ESTROGENIC SUBSTANCE-ESTRONE; OESTRONE; Oestrin; Oestroform;Oestronum; Oestrone [Steroidal oestrogens]; Oestrone, Estrone; Oestroperos; Ovex (tablets); Ovifollin; Penncap M; Perlatan; Solliculin; Theelin; Thelestrin; Thelykinin; Thynestron; Tokokin; Unden; Unden (pharmaceutical); Unden (pharmaceutical) (VAN); Unigen; Vortech Brand of Estrone; Wehgen; Wynestron; WynestronPencap M;CMC_13458; [2,4,6,7-3H]-E1
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Postmenopausal disorder [ICD11: GA30.Z] | Approved | [1] | |||
| Therapeutic Class |
Antimenopausal Agents
|
|||||
| Structure |
|
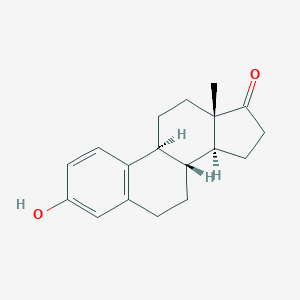 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C18H22O2
|
|||||
| Canonical SMILES |
CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O
|
|||||
| InChI |
InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
|
|||||
| InChIKey |
DNXHEGUUPJUMQT-CBZIJGRNSA-N
|
|||||
| CAS Number |
CAS 53-16-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 270.4 | Topological Polar Surface Area | 37.3 | ||
| Heavy Atom Count | 20 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
3.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
11466942
, 11468062
, 11486713
, 14714953
, 14750534
, 14774967
, 17405076
, 24278427
, 24715020
, 24870303
, 24894399
, 26512283
, 26751561
, 26751562
, 2687
, 29224898
, 3133155
, 3751
, 46505916
, 46517467
, 47213246
, 47275539
, 47424227
, 47646545
, 47646546
, 47646547
, 47720600
, 47943749
, 48094530
, 48243347
, 48318399
, 48421873
, 49684201
, 49699003
, 49965933
, 50104169
, 50104170
, 50124146
, 50124147
, 50124148
, 50967133
, 53777619
, 53787846
, 613807
, 7847135
, 7979191
, 8144215
, 8153628
, 841396
, 855919
|
|||||
| ChEBI ID |
ChEBI:17263
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Estrone was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | P-glycoprotein (P-gp/MDR1)-mediated efflux of sex-steroid hormones and modulation of P-gp expression in vitro. Pharm Res. 2004 Jul;21(7):1284-93. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
