Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00537
|
|||||
| Drug Name |
Ceftibuten
|
|||||
| Synonyms |
(+)-(6R,7R)-7-((Z)-2-(2-Amino-4-thiazolyl)-4-carboxycrotonamido)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(E)-2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(Z)-2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-4-carboxybut-2-enoyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-[[(E)-2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-[[(Z)-2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-[[2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7432-S; 7beta-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-4-carboxybut-2-enoyl]amino}-3,4-didehydrocepham-4-carboxylic acid; Antibiotic 7432S; CETB; Cedax; Cedax (TN); Ceftem; Ceftibuten [USAN:INN:BAN]; Ceftibuten(USAN/INN); Ceftibutene; Ceftibutene [INN-French]; Ceftibuteno; Ceftibuteno [INN-Spanish]; Ceftibutenum; Ceftibutenum [INN-Latin]; Cephalosporin 7432-S; Cephem; Ceprifran; Cis-Ceftibutin; Cis-ceftibuten; Isocef; Keimax; S 7432; Sch 39720; Sch-39720; Trans-Ceftibuten
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Chronic bronchitis [ICD11: CA20.1] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
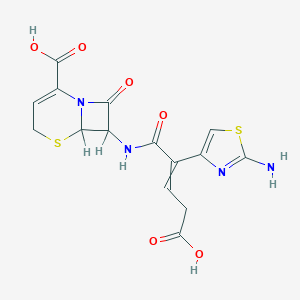 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C15H14N4O6S2
|
|||||
| Canonical SMILES |
C1C=C(N2C(S1)C(C2=O)NC(=O)C(=CCC(=O)O)C3=CSC(=N3)N)C(=O)O
|
|||||
| InChI |
InChI=1S/C15H14N4O6S2/c16-15-17-7(5-27-15)6(1-2-9(20)21)11(22)18-10-12(23)19-8(14(24)25)3-4-26-13(10)19/h1,3,5,10,13H,2,4H2,(H2,16,17)(H,18,22)(H,20,21)(H,24,25)
|
|||||
| InChIKey |
UNJFKXSSGBWRBZ-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 97519-39-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 410.4 | Topological Polar Surface Area | 217 | ||
| Heavy Atom Count | 27 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 10 | |||
| XLogP |
-0.3
|
|||||
| PubChem CID | ||||||
| PubChem SID | ||||||
| ChEBI ID |
CHEBI:3510
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [2] | |
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [3] | ||
| References | ||||||
| 1 | Ceftibuten was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Increased protein level of PEPT1 intestinal H+-peptide cotransporter upregulates absorption of glycylsarcosine and ceftibuten in 5/6 nephrectomized rats. Am J Physiol Gastrointest Liver Physiol. 2005 Apr;288(4):G664-70. | |||||
| 3 | Transport characteristics of a novel peptide transporter 1 substrate, antihypotensive drug midodrine, and its amino acid derivatives. J Pharmacol Exp Ther. 2006 Jul;318(1):455-60. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
