Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00539
|
|||||
| Drug Name |
Cefmetazole
|
|||||
| Synonyms |
(6R,7S)-7-(2-((Cyanomethyl)thio)acetamido)-7-methoxy-3-(((1-methyl-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; (6R,7S)-7-(2-((Cyanomethyl)thio)acetamido)-7-methoxy-3-(((1-methyl-1H-tetrazol-5-yl)thiomethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, sodium salt; (6R,7S)-7-({[(cyanomethyl)sulfanyl]acetyl}amino)-7-methoxy-3-{[(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7S)-7-({[(cyanomethyl)thio]acetyl}amino)-7-(methyloxy)-3-{[(1-methyl-1H-tetrazol-5-yl)thio]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7S)-7-[[2-(cyanomethylsulfanyl)acetyl]amino]-7-methoxy-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 6beta-{[(cyanomethyl)sulfanyl]acetamido}-6alpha-methoxy-3-{[(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl}ceph-3-em-4-carboxylic acid; CMZ; CS 1170; CS-1170; Cefmetazole (USP/INN); Cefmetazole Monosodium Salt; Cefmetazole [USAN:INN]; Cefmetazolo; Cefmetazolo [INN-Spanish]; Cefmetazolum; Cefmetazolum [INN-Latin]; SKF 83088; U 72791; U-72791A
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Urinary tract and skin bacteria infections [ICD11: GC08] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
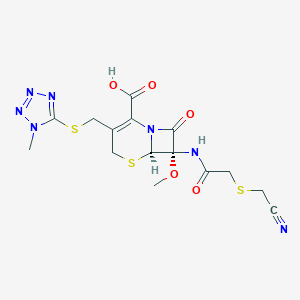 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C15H17N7O5S3
|
|||||
| Canonical SMILES |
CN1C(=NN=N1)SCC2=C(N3C(C(C3=O)(NC(=O)CSCC#N)OC)SC2)C(=O)O
|
|||||
| InChI |
InChI=1S/C15H17N7O5S3/c1-21-14(18-19-20-21)30-6-8-5-29-13-15(27-2,17-9(23)7-28-4-3-16)12(26)22(13)10(8)11(24)25/h13H,4-7H2,1-2H3,(H,17,23)(H,24,25)/t13-,15+/m1/s1
|
|||||
| InChIKey |
SNBUBQHDYVFSQF-HIFRSBDPSA-N
|
|||||
| CAS Number |
CAS 56796-20-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 471.5 | Topological Polar Surface Area | 239 | ||
| Heavy Atom Count | 30 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 12 | |||
| XLogP |
-0.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10303
, 104338473
, 11466728
, 11467848
, 11486516
, 123087792
, 124766309
, 126625684
, 126686221
, 131319170
, 134224281
, 134338424
, 135002546
, 135882605
, 136357157
, 137006381
, 140396436
, 152035638
, 15479323
, 160963622
, 162173144
, 164788224
, 175442148
, 179150673
, 184544918
, 198970111
, 223674319
, 226513671
, 251916490
, 251917837
, 252347145
, 252449370
, 34707577
, 46504461
, 47217057
, 47217058
, 47440545
, 47440546
, 48259496
, 48415716
, 50050974
, 50294685
, 57312636
, 58106756
, 632166
, 7847973
, 7978876
, 8177398
, 92251462
, 92712300
|
|||||
| ChEBI ID |
ChEBI:3489
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [2] | |
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Cefmetazole was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Screening of antibiotics that interact with organic anion-transporting polypeptides 1B1 and 1B3 using fluorescent probes. Biol Pharm Bull. 2011;34(3):389-95. | |||||
| 3 | Intestinal transport of beta-lactam antibiotics: analysis of the affinity at the H+/peptide symporter (PEPT1), the uptake into Caco-2 cell monolayers and the transepithelial flux. Pharm Res. 1999 Jan;16(1):55-61. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
