Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00544
|
|||||
| Drug Name |
Cephradine
|
|||||
| Synonyms |
(6R,7R)-7-((R)-2-Amino-2-(1,4-cyclohexadien-1-yl)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2R)-2-amino-2-cyclohexa-1,4-dien-1-ylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2R)-2-amino-2-cyclohexa-1,4-dien-1-ylacetyl]amino}-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-(D-2-Amino-2-(1,4-cyclohexadien-1-yl)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)-oct-2-ene-2-caboxylic acid; 7-(D-2-Amino-2-(1,4-cyclohexadienyl)acetamide)desacetoxycephalosporanicacid; 7beta-[(2R)-2-(cyclohexa-1,4-dienyl)-2-phenylacetamido]-3-methyl-3,4-didehydrocepham-4-carboxylic acid; Anspor; Anspor (TN); CEPHRADINE SODIUM; Cefradin; Cefradina; Cefradina [INN-Spanish]; Cefradine (JAN/INN); Cefradinum; Cefradinum [INN-Latin]; Cephradin; Cephradine; Cephradine (USP); Cephradine (anhydrous); Cephradine [USAN:BAN]; Eskacef; SK&F D-39304; SK-D-39304; SKF D 39304; SQ 11436; SQ-11436; SQ-22022; Sefril; VELOSEF 125; VELOSEF 250; VELOSEF 500; Velosef; Velosef (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Streptococcus pneumoniae infections [ICD11: CA40.0Y] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
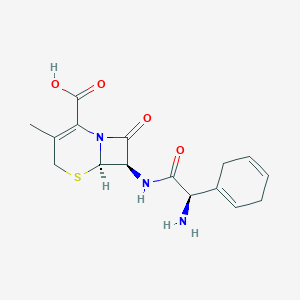 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C16H19N3O4S
|
|||||
| Canonical SMILES |
CC1=C(N2C(C(C2=O)NC(=O)C(C3=CCC=CC3)N)SC1)C(=O)O
|
|||||
| InChI |
InChI=1S/C16H19N3O4S/c1-8-7-24-15-11(14(21)19(15)12(8)16(22)23)18-13(20)10(17)9-5-3-2-4-6-9/h2-3,6,10-11,15H,4-5,7,17H2,1H3,(H,18,20)(H,22,23)/t10-,11-,15-/m1/s1
|
|||||
| InChIKey |
RDLPVSKMFDYCOR-UEKVPHQBSA-N
|
|||||
| CAS Number |
CAS 38821-53-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 349.4 | Topological Polar Surface Area | 138 | ||
| Heavy Atom Count | 24 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
0.4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103590704
, 104133787
, 104328337
, 11335914
, 11361153
, 11462125
, 117600507
, 124766238
, 124894242
, 126625602
, 126656737
, 134222860
, 134337749
, 135001466
, 136212369
, 136367999
, 137006375
, 137263524
, 140396423
, 144115808
, 144207037
, 15076177
, 152100549
, 160849687
, 160964630
, 162175008
, 164788183
, 170465355
, 172914782
, 174006223
, 175442153
, 175607974
, 178101532
, 179116612
, 24278342
, 34679326
, 46505082
, 47365212
, 48415745
, 49836646
, 50050883
, 50880148
, 57312171
, 7847330
, 7978895
, 8149240
, 8175363
, 9114
, 92712397
, 99301827
|
|||||
| ChEBI ID |
ChEBI:3547
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [2] | |
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [3] | ||
| References | ||||||
| 1 | Cephradine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Recognition of beta-lactam antibiotics by rat peptide transporters, PEPT1 and PEPT2, in LLC-PK1 cells. Am J Physiol. 1997 Nov;273(5 Pt 2):F706-11. | |||||
| 3 | Transport characteristics of a novel peptide transporter 1 substrate, antihypotensive drug midodrine, and its amino acid derivatives. J Pharmacol Exp Ther. 2006 Jul;318(1):455-60. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
