Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00546
|
|||||
| Drug Name |
Cefixime
|
|||||
| Synonyms |
(6R,7R)-7-({(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(carboxymethoxy)imino]acetyl}amino)-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(carboxymethyloxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-{[(carboxymethyl)oxy]imino}acetyl]amino}-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6r,7r)-7-[-2-(2-amino-thiazol-4-yl)-2-carboxymethoxyimino-acetylamino]-8-oxo-3-vinyl-5-thia-1-aza-b; 7beta-{(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(carboxymethoxy)imino]acetamido}-3-ethenyl-3,4-didehydrocepham-4-carboxylic acid; CFIX; CL-284635; Cefixima; Cefixime (JP15/USP/INN); Cefiximum; Denvar; FK-027; FR-17027; Necopen; Ofex (TN); Suprax (TN); Tricef
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Streptococcus bacterial infections [ICD11: 1C41] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
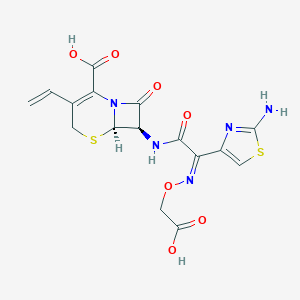 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C16H15N5O7S2
|
|||||
| Canonical SMILES |
C=CC1=C(N2C(C(C2=O)NC(=O)C(=NOCC(=O)O)C3=CSC(=N3)N)SC1)C(=O)O
|
|||||
| InChI |
InChI=1S/C16H15N5O7S2/c1-2-6-4-29-14-10(13(25)21(14)11(6)15(26)27)19-12(24)9(20-28-3-8(22)23)7-5-30-16(17)18-7/h2,5,10,14H,1,3-4H2,(H2,17,18)(H,19,24)(H,22,23)(H,26,27)/b20-9-/t10-,14-/m1/s1
|
|||||
| InChIKey |
OKBVVJOGVLARMR-QSWIMTSFSA-N
|
|||||
| CAS Number |
CAS 79350-37-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 453.5 | Topological Polar Surface Area | 238 | ||
| Heavy Atom Count | 30 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 12 | |||
| XLogP |
-0.7
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103567682
, 104133792
, 114149638
, 11654000
, 117664404
, 12012851
, 121363201
, 124800909
, 126625637
, 126656766
, 126667588
, 134337735
, 135601356
, 135693789
, 137100974
, 137240061
, 143493269
, 14833440
, 151994359
, 152100554
, 152242797
, 15404783
, 162258044
, 163426477
, 164196526
, 174560966
, 175265490
, 175611868
, 179116601
, 184812415
, 198991919
, 203356010
, 39384388
, 46508684
, 47288991
, 47362877
, 47957436
, 48415714
, 49699091
, 50070784
, 50124237
, 57362078
, 7847324
, 78846797
, 7978875
, 85148376
, 85661978
, 85788072
, 9098
, 92712349
|
|||||
| ChEBI ID |
CHEBI:472657
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [2] | |
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [3] | ||
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [3] | ||
| References | ||||||
| 1 | Cefixime was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Flavonoids with epidermal growth factor-receptor tyrosine kinase inhibitory activity stimulate PEPT1-mediated cefixime uptake into human intestinal epithelial cells. J Pharmacol Exp Ther. 2001 Oct;299(1):351-7. | |||||
| 3 | Transport characteristics of a novel peptide transporter 1 substrate, antihypotensive drug midodrine, and its amino acid derivatives. J Pharmacol Exp Ther. 2006 Jul;318(1):455-60. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
