Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00577
|
|||||
| Drug Name |
Cefazolin
|
|||||
| Synonyms |
(6R, 7R)-3-[[(5-Methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[[1H-tetrazol-1-yl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-(((5-Methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-7-(2-(1H-tetrazol-1-yl)acetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; (6R,7R)-3-[(5-methyl-1,3,4-thiadiazol-2-yl)sulfanylmethyl]-8-oxo-7-[[2-(tetrazol-1-yl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-{[(5-methyl-1,3,4-thiadiazol-2-yl)sulfanyl]methyl}-8-oxo-7-[(1H-tetrazol-1-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-{[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl}-8-oxo-7-[(1H-tetrazol-1-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R-trans)-3-(((5-Methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-7-(((1H-tetrazol-1-yl)acetyl)-amino)-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; 3-{[(5-methyl-1,3,4-thiadiazol-2-yl)sulfanyl]methyl}-7beta-[(1H-tetrazol-1-ylacetyl)amino]-3,4-didehydrocepham-4-carboxylic acid; 7-(1-(1H-)-Tetrazolylacetamido)-3-(2-(5-methyl-1,3,4-thiadiazolyl)thiomethyl)delta3-cephem-4-carboxylic acid; Ancef (TN); CEZ; Cefacidal (TN); Cefamezin; Cefamezin (TN); Cefamezine; Cefazolin (USP); Cefazolin [USAN:INN]; Cefazolin(usp); Cefazolina; Cefazolina [INN-Spanish]; Cefazoline; Cefazoline [INN-French]; Cefazolinum; Cefazolinum [INN-Latin]; Cefrina (TN); Cephamezine; Cephazolidin; Cephazolin; Cephazolin Sodium; Cephazoline; Elzogram; Elzogram (TN); Faxilen (TN); Gramaxin (TN); Kefazol (TN); Kefol (TN); Kefzol (TN); Kefzolan (TN); Kezolin (TN); Novaporin (TN); Zolicef (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Methicillin-susceptible staphylococcus aureus [ICD11: 1B74.0] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
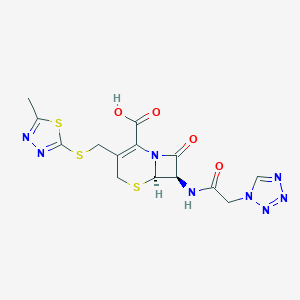 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C14H14N8O4S3
|
|||||
| Canonical SMILES |
CC1=NN=C(S1)SCC2=C(N3C(C(C3=O)NC(=O)CN4C=NN=N4)SC2)C(=O)O
|
|||||
| InChI |
InChI=1S/C14H14N8O4S3/c1-6-17-18-14(29-6)28-4-7-3-27-12-9(11(24)22(12)10(7)13(25)26)16-8(23)2-21-5-15-19-20-21/h5,9,12H,2-4H2,1H3,(H,16,23)(H,25,26)/t9-,12-/m1/s1
|
|||||
| InChIKey |
MLYYVTUWGNIJIB-BXKDBHETSA-N
|
|||||
| CAS Number |
CAS 25953-19-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 454.5 | Topological Polar Surface Area | 235 | ||
| Heavy Atom Count | 29 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 12 | |||
| XLogP |
-0.4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103510684
, 104133789
, 104231690
, 104315593
, 11335189
, 11360428
, 11362960
, 11365522
, 11368084
, 11371259
, 11373873
, 11376246
, 11461400
, 11466764
, 11467884
, 11484775
, 11486367
, 11488956
, 11490146
, 11492082
, 11493920
, 117600526
, 124766146
, 14833488
, 15008581
, 29215418
, 34675123
, 46506123
, 47193754
, 47290958
, 47440062
, 47440063
, 47440064
, 47810578
, 48110278
, 48259043
, 48334299
, 48415713
, 50051012
, 56314760
, 57311487
, 602960
, 75509242
, 7849358
, 7978873
, 81093146
, 8173059
, 85663279
, 9097
, 92714647
|
|||||
| ChEBI ID |
CHEBI:474053
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [2] | |
| OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [3] | ||
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [3] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [4] | ||
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [5] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | MRP4 | Transporter Info | Km = 81 microM | Human embryonic kidney cells (HEK293)-MRP4 | [2] | |
| References | ||||||
| 1 | Cefazolin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Oral availability of cefadroxil depends on ABCC3 and ABCC4. Drug Metab Dispos. 2012 Mar;40(3):515-21. | |||||
| 3 | Expression levels of renal organic anion transporters (OATs) and their correlation with anionic drug excretion in patients with renal diseases. Pharm Res. 2004 Jan;21(1):61-7. | |||||
| 4 | Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011 Mar;63(1):157-81. | |||||
| 5 | Three-dimensional quantitative structure-activity relationship analyses of beta-lactam antibiotics and tripeptides as substrates of the mammalian H+/peptide cotransporter PEPT1. J Med Chem. 2005 Jun 30;48(13):4410-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
