Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00590
|
|||||
| Drug Name |
Trimethoprim
|
|||||
| Synonyms |
AZT + TMP/SMX (mixture) combination; Abaprim; Alcorim-F; Alprim; Anitrim; Antrima; Antrimox; Apo-Sulfatrim; BW 56-72; BW 5672; BW-56-72; Bacdan; Bacidal; Bacide; Bacin; Bacta; Bacterial; Bacterial [Antibiotic]; Bacticel; Bactin; Bactoprim; Bactramin; Bencole; Bethaprim; Biosulten; Briscotrim; Chemotrin; Cidal; Co-Trimoxizole; Colizole; Colizole DS; Component of Bactrim; Component of Septra; Conprim; Cotrimel; Deprim; Duocide; Esbesul; Espectrin; Euctrim; Exbesul; Fermagex; Fortrim; Futin; Idotrim; Ikaprim; Instalac; KSC-4-158; KUC103659N; Kombinax; Lagatrim; Lagatrim Forte; Lastrim; Methoprim; Metoprim; Monoprim; Monotrim; Monotrim (TN); Monotrimin; NIH 204; NIH 204 (VAN); Novotrimel; Omstat; Oraprim; Pancidim; Polytrim; Priloprim; Primosept; Primsol; Proloprim; Proloprim (TN); Protrin; Purbal; Resprim; ResprimForte; Roubac; Roubal; Salvatrim; Septrin DS; Septrin Forte; Septrin S; Setprin; Sinotrim; Smz-Tmp; Stopan; Streptoplus; Sugaprim; Sulfamar; Sulfamethoprim; Sulfamethoxazole & Trimethoprim; Sulfoxaprim; Sulmeprim; Sulthrim; Sultrex; Syraprim; T 7883; TCMDC-125538; TMP; TMP/SMX (MIXTURE)); Tiempe; Tmp-Ratiopharm; Toprim; Trimanyl; Trimeth/Sulfa; Trimethioprim; Trimethopim(TMP); Trimethoprim & VRC3375; Trimethoprim (JAN/USP/INN); Trimethoprim [USAN:BAN:INN:JAN]; Trimethoprime; Trimethoprime [INN-French]; Trimethoprimum; Trimethoprimum [INN-Latin]; Trimethopriom; Trimetoprim; Trimetoprim [DCIT]; Trimetoprim [Polish]; Trimetoprima; Trimetoprima [INN-Spanish]; Trimexazole; Trimexol; Trimez-IFSA; Trimezol; Trimogal; Trimono; Trimopan; Trimpex; Trimpex (TN); Trimpex 200; Triprim; Triprim (TN); Trisul; Trisulcom; Trisulfam; Trisural; U-Prin; Uretrim; Uro-D S; Urobactrim; Utetrin; Velaten; WR 5949; Wellcoprim; Wellcoprin; Xeroprim; Zamboprim
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Urinary tract infections [ICD11: GC08] | Approved | [1] | |||
| Therapeutic Class |
Antiinfective Agents
|
|||||
| Structure |
|
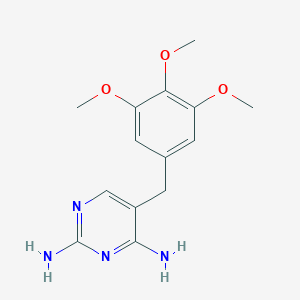 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C14H18N4O3
|
|||||
| Canonical SMILES |
COC1=CC(=CC(=C1OC)OC)CC2=CN=C(N=C2N)N
|
|||||
| InChI |
InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18)
|
|||||
| InChIKey |
IEDVJHCEMCRBQM-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 738-70-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 290.32 | Topological Polar Surface Area | 106 | ||
| Heavy Atom Count | 21 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
0.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321479
, 10534335
, 11111923
, 11111924
, 11113621
, 11335764
, 11361003
, 11363061
, 11365623
, 11368185
, 11371565
, 11374002
, 11376347
, 11461975
, 11466236
, 11467356
, 11484610
, 11485852
, 11488753
, 11490459
, 11492187
, 11493981
, 14824824
, 17389524
, 17405825
, 22391545
, 24278201
, 24870616
, 24889615
, 25623645
, 26611964
, 26680042
, 26697109
, 26704777
, 26747124
, 26747125
, 26751820
, 3146445
, 406777
, 4752045
, 5066
, 604810
, 7847213
, 7890832
, 7980835
, 8027414
, 8149571
, 8153433
, 837167
, 857743
|
|||||
| ChEBI ID |
ChEBI:45924
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Trimethoprim was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42. | |||||
| 3 | Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev. 2012 Jan;64(1):95-109. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
