Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00623
|
|||||
| Drug Name |
Mefloquine
|
|||||
| Synonyms |
(+)-(11R,2'S)-erythro-Mefloquine; (+)-Mefloquine; (+)-Threo-Mefloquine; (-)-(11S,2'R)-erythro-Mefloquine; (-)-Mefloquine; (-)-Threo-Mefloquine; (DL-erythro-alpha-2-Piperidyl-2,8-bis(trifluoromethyl)-4-quinolinemethanol; (R)-[2,8-bis(trifluoromethyl)quinolin-4-yl]-[(2R)-piperidin-2-yl]methanol; (R)-[2,8-bis(trifluoromethyl)quinolin-4-yl]-[(2S)-piperidin-2-yl]methanol; (S)-[2,8-bis(trifluoromethyl)quinolin-4-yl]-[(2R)-piperidin-2-yl]methanol; (S)-[2,8-bis(trifluoromethyl)quinolin-4-yl]-[(2S)-piperidin-2-yl]methanol; (S)-[2,8-bis(trifluoromethyl)quinolin-4-yl][(2R)-piperidin-2-yl]methanol; Alpha-2-Piperidinyl-2,8-bis(trifluoromethyl)-4-quinolinemethanol; Alpha-2-Piperidyl-2,8-bis(trifluoromethyl)quinoline-4-methanol; Erthro-.alpha.-[2-piperidyl]-2,8-bis[trifluoromethyl]-4-quinolinemethanol; Lariam; Lariam (Hydrochloride); Lariam (TN); Mefaquin (TN); Mefloquin; Mefloquina; Mefloquina [INN-Spanish]; Mefloquine (USAN/INN); Mefloquine [USAN:INN:BAN]; Mefloquinone; Mefloquinum; Mefloquinum [INN-Latin]; Mephloquine; RO 13-7224; RO 13-7225; RTI1169-1-1; RTI1172-1-1; RTI1173-1-1; RTI1174-1-1; RTI1188-1-1; RTI1189-1-1; Racemic mefloquine; Ro 21-5998; Ro 21-5998 (Hydrochloride); Ro 215998; Ro-21-5998-001; SPB-80406; WR 142490; WR-142,490; WR-142490; WR-177,602; [2,8-bis(trifluoromethyl)quinolin-4-yl]-piperidin-2-ylmethanol
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Malaria [ICD11: 1F40] | Approved | [1] | |||
| Therapeutic Class |
Antimalarials
|
|||||
| Structure |
|
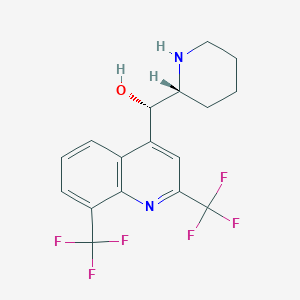 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C17H16F6N2O
|
|||||
| Canonical SMILES |
C1CCNC(C1)C(C2=CC(=NC3=C2C=CC=C3C(F)(F)F)C(F)(F)F)O
|
|||||
| InChI |
InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1
|
|||||
| InChIKey |
XEEQGYMUWCZPDN-DOMZBBRYSA-N
|
|||||
| CAS Number |
CAS 53230-10-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 378.31 | Topological Polar Surface Area | 45.2 | ||
| Heavy Atom Count | 26 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 9 | |||
| XLogP |
3.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103446060
, 104040016
, 104335053
, 124892361
, 135004437
, 135240375
, 135611150
, 135727482
, 137236385
, 140239432
, 14877790
, 175269604
, 178101069
, 179116766
, 223533437
, 223712526
, 226410988
, 243866056
, 252401319
, 252655885
, 26755467
, 29216385
, 34706437
, 47206659
, 49681220
, 50042965
, 50071333
, 51051233
, 53788688
, 56310966
, 56313823
, 57312460
, 603007
, 8176741
, 85154870
, 93166754
, 93167184
, 9835
|
|||||
| ChEBI ID |
CHEBI:63684
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Doxycycline was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004 Jan;75(1):13-33. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
