Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00672
|
|||||
| Drug Name |
Emtricitabine
|
|||||
| Synonyms |
(-)-(2R,5S)-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; (-)-.beta.-L-FTC; (-)-2',3'-Dideoxy-5-fluoro-3'-thiacytidine; (-)-2'-Deoxy-5-fluoro-3'-thiacytidine; (-)-FTC; (-)-beta-2',3'-dideoxy-5-fluoro-3'-thiacytidine; (-)-cis-4-amino-5-fluoro-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one; (2R-cis)-4-Amino-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-2(1H)-pyrimidinone; 1-(2-(Hydroxymethyl)oxathiolan-5-yl)-5-fluorocytosine; 2',3',5-FTC; 2',3'-Dideoxy-5-fluoro-3'-thiacytidine; 2'-Deoxy-5-fluoro-3'-oxa-4'-thiocytidine; 2'-Deoxy-5-fluoro-3'-thiacytidine; 2-FTC; 3'-Thia-2'.3'-dideoxy-5-fluorocytidine; 4-Amino-5-fluoro-1-[(2R,5S)-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidinone; 4-amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one; 4-amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one; 5-Fluoro-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine; 5-Fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine; 5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)[1,3]oxathiolan-5-yl]cytosine; 524W91; BW 1592; BW 524W91; BW-524W91; BW524W91; Beta-L-(-)-(2R,5S)-5-Fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; Beta-L-2',3'-dideoxy-5-fluoro-3'-thiacytidine; Coviracil; Coviracil (TN); Coviracil(TM); DOTFC; DRG-0208; Emtricitabine (JAN/USAN/INN); Emtriva; Emtriva(TM); FTC; RCV; Racivir
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Hepatitis B virus infection [ICD11: 1E51.0, 1E50.1] | Approved | [1] | |||
| Human immunodeficiency virus infection [ICD11: 1C62.Z] | Approved | [1] | ||||
| Therapeutic Class |
Anti-HIV Agents
|
|||||
| Structure |
|
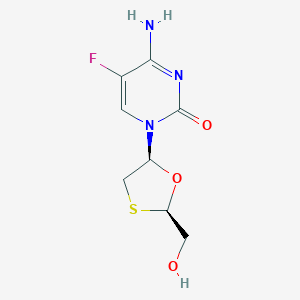 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H10FN3O3S
|
|||||
| Canonical SMILES |
C1C(OC(S1)CO)N2C=C(C(=NC2=O)N)F
|
|||||
| InChI |
InChI=1S/C8H10FN3O3S/c9-4-1-12(8(14)11-7(4)10)5-3-16-6(2-13)15-5/h1,5-6,13H,2-3H2,(H2,10,11,14)/t5-,6+/m0/s1
|
|||||
| InChIKey |
XQSPYNMVSIKCOC-NTSWFWBYSA-N
|
|||||
| CAS Number |
CAS 143491-57-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 247.25 | Topological Polar Surface Area | 113 | ||
| Heavy Atom Count | 16 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
-0.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103232542
, 104178758
, 104321867
, 11528728
, 117506117
, 118046007
, 118313743
, 12014747
, 126592961
, 126653602
, 126654249
, 126665394
, 127310207
, 127310208
, 129727356
, 134223773
, 134338341
, 135018286
, 135022892
, 135590997
, 136947986
, 137006539
, 142372786
, 144205746
, 14749730
, 14847619
, 151992517
, 152034369
, 160826182
, 160964220
, 162792750
, 163388307
, 163621100
, 24875080
, 26757984
, 43118210
, 46507606
, 48424313
, 49830960
, 50064421
, 57314168
, 582989
, 600446
, 71814561
, 76230216
, 7848262
, 8187110
, 92308841
, 92729801
, 99313695
|
|||||
| ChEBI ID |
CHEBI:31536
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MATE1 | Transporter Info | Multidrug and toxin extrusion protein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Emtricitabine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Emtricitabine is a substrate of MATE1 but not of OCT1, OCT2, P-gp, BCRP or MRP2 transporters. Xenobiotica. 2017 Jan;47(1):77-85. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
