Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00705
|
|||||
| Drug Name |
Astemizole
|
|||||
| Synonyms |
1-(p-Fluorobenzyl)-2-((1-(p-methoxyphenethyl)-4-piperidyl)amino)benzimidazole; AlacanBrand of Astemizole; Alermizol; Alonga Brand of Astemizole; Alonga, Astemizol; Astemina; Astemisan; Astemisol; Astemison; Astemizol; Astemizol Alonga; Astemizol [German]; Astemizol [INN-Spanish]; Astemizol ratiopharm; Astemizole (JAN/USP/INN); Astemizole Alacan Brand; Astemizole Alonga Brand; Astemizole Byk Brand; Astemizole Diba Brand; Astemizole Elfar Brand; Astemizole Esteve Brand; Astemizole Fustery Brand; Astemizole ICN Brand; Astemizole Janssen Brand; Astemizole Lesvi Brand; Astemizole McNeil Brand; Astemizole Medinsa Brand; Astemizole Merck Brand; Astemizole Senosiain Brand; Astemizole Septa Brand; Astemizole Smaller Brand; Astemizole Urbion Brand; Astemizole Vita Brand; Astemizole [USAN:BAN:INN]; Astemizole ratiopharm Brand; Astemizolum; Astemizolum [INN-Latin]; Astesen; Byk Brand of Astemizole; Diba Brand of Astemizole; Elfar Brand of Astemizole; Emdar; Esmacen;Fustermizol; Esteve Brand of Astemizole; Fustery Brand of Astemizole; GNF-PF-2461; HISMANAL (TN); Hestazol; Hestazol, Kelp, Laridal, Retolen, Wareezol, HSBD 6799, BRN 4830190; Hismanal; Histamen; Histaminos; Histazol; Hubermizol; ICN Brand of Astemizole; Janssen Brand of Astemizole; Kelp; Laridal; Lesvi Brand of Astemizole; MJD-30; McNeil Brand of Astemizole; Medinsa Brand of Astemizole; Merck Brand of Astemizole; Metodih; Metodik; Nono-Nastizol A; Novo-mastizol A; Paralergin; R 42512; R 43 512; R-43-512; R-43512; R43512; Ratiopharm Brand of Astemizole; Ratiopharm, Astemizol; Reig Jofre Brand of Astemizole; Retolen; Rifedot; Rimbol; Romadin; Senosiain Brand of Astemizole; Septa Brand of Astemizole; Simprox; Smaller Brand of Astemizole; Urbion Brand of Astemizole; Urdrim; Vita Brand of Astemizole; Wareezol; Waruzol; [3H]Astemizole
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Allergic rhinitis [ICD11: CA08.0] | Withdrawn | [1] | |||
| Therapeutic Class |
Antiallergic Agents
|
|||||
| Structure |
|
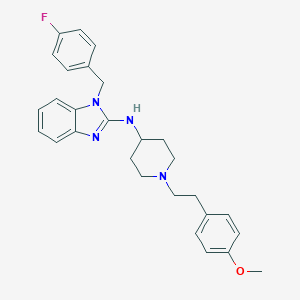 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C28H31FN4O
|
|||||
| Canonical SMILES |
COC1=CC=C(C=C1)CCN2CCC(CC2)NC3=NC4=CC=CC=C4N3CC5=CC=C(C=C5)F
|
|||||
| InChI |
InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31)
|
|||||
| InChIKey |
GXDALQBWZGODGZ-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 68844-77-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 458.6 | Topological Polar Surface Area | 42.3 | ||
| Heavy Atom Count | 34 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321136
, 11112828
, 11335214
, 11360453
, 11363342
, 11365904
, 11368466
, 11372003
, 11374824
, 11376628
, 11461425
, 11466164
, 11467284
, 11485540
, 11485887
, 11489548
, 11490887
, 11493037
, 11494262
, 11528621
, 11538010
, 12013338
, 14760289
, 22391432
, 26613163
, 26680817
, 26746979
, 26746980
, 26751496
, 26751497
, 29221421
, 4266413
, 459037
, 46487928
, 46508569
, 47216611
, 47440072
, 47515146
, 47736292
, 47810587
, 47885241
, 48034934
, 48259050
, 5313663
, 7847301
, 7978732
, 8150150
, 8151518
, 855746
, 9050
|
|||||
| ChEBI ID |
CHEBI:2896
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Comparative tolerability of second generation antihistamines. Drug Saf. 1999 May;20(5):385-401. | |||||
| 2 | Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev. 2012 Jan;64(1):95-109. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
