Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00707
|
|||||
| Drug Name |
Trichloromethiazide
|
|||||
| Synonyms |
(+-)-6-Chloro-3-(dichloromethyl)-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-3-(dichloromethyl)-3,4-dihydro-, 1,1-dioxide; 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-3-(dichloromethyl)-3,4-dihydro-,1,1-dioxide; 3-(Dichloromethyl)-6-chloro-7-sulfamoyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide; 3-(Dichloromethyl)-6-chloro-7-sulfamoyl-3,4-dihydro-1,2,4-benzothiadiazine-1,1-dioxide; 3-Dichloromethyl-6-chloro-7-sulfamoyl-3,4-dihydro-1,2,4-benzothiadiazine-1,1-dioxide; 3-Dichloromethyl-6-chloro-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide; 3-Dichloromethyl-6-chloro-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine1,1-dioxide; 3-Dichloromethylhydrochlorothiazide; 6-Chloro-3-(dichloromethyl)-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 6-Chloro-3-(dichloromethyl)-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide1,1-dioxide; 6-Chloro-3-(dichloromethyl)3,4-dihydro-7-sulfamoyl-1,2,4-benzothiadiazine-1,1-dioxide; 6-Chloro-3-(dichloromethyl)3,4-dihydro-7-sulfamyl-1,2,4-benzothiadiazine-1,1-dioxide; 6-Chloro-3-[dichloromethyl]-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxide; 6-Chloro-3-dichloromethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-Dioxide; 6-Chloro-3-dichloromethyl-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine1,1-dioxide; Achletin; Achletin (TN); Anistadin; Aponorin; Aquazide; Carvacron; Chlopolidine; Ciba 7057-Su; Cretonin; Diu-Hydrin; Diu-Hydrin (TN); Diurazida; Diurese; Diuroral;Esmarin; Eurinol; Fluitran; Flurese; Flurese (VAN); Flutra; Gangesol; Hydrotrichlorothiazide; Intromene; Isestran; Jones Brand of Trichloromethiazide; Kubacron; Metahydrin; Metatensin; Nakva; Naqua; Naqua (TN); Naquasone; Salurin (wadel); Schebitran; Schering Brand of Trichlormethiazide; Tachionin; Tolcasone; Trichlordiuride; Trichlorex; Trichlormas; Trichlormetazid; Trichlormethiazid; Trichlormethiazide (JP15/USP/INN); Trichlormethiazide W/ Reserpine; Trichlormethiazide [INN:JAN]; Trichlormethiazidum; Trichlormethiazidum [INN-Latin]; Trichloromethiadiazide; Trichloromethiazide; Trichloromethiazide, 6; Triclordiuride; Triclormetiazida; Triclormetiazida[INN-Spanish]; Triclormetiazide; Triclormetiazide [DCIT]; Triclormetiazide [Italian]; Triflumen (TN); Triflumen;American Urologicals Brand of Trichloromethiazide
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Edema associated with heart failure [ICD11: BD10.Z] | Approved | [1] | |||
| Therapeutic Class |
Antihypertensive Agents
|
|||||
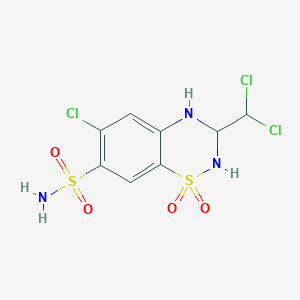
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H8Cl3N3O4S2
|
|||||
| Canonical SMILES |
C1=C2C(=CC(=C1Cl)S(=O)(=O)N)S(=O)(=O)NC(N2)C(Cl)Cl
|
|||||
| InChI |
InChI=1S/C8H8Cl3N3O4S2/c9-3-1-4-6(2-5(3)19(12,15)16)20(17,18)14-8(13-4)7(10)11/h1-2,7-8,13-14H,(H2,12,15,16)
|
|||||
| InChIKey |
LMJSLTNSBFUCMU-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 133-67-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 380.7 | Topological Polar Surface Area | 135 | ||
| Heavy Atom Count | 20 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
0.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321929
, 10506863
, 108999
, 11335541
, 11360780
, 11363414
, 11365976
, 11368538
, 11372245
, 11373972
, 11376700
, 11455198
, 11461752
, 11466853
, 11467973
, 11484652
, 11486406
, 11488764
, 11491188
, 11492182
, 11494334
, 14780142
, 24899924
, 26611959
, 26747211
, 26747212
, 29224600
, 46508880
, 47440176
, 47588924
, 47662203
, 47810669
, 47885335
, 47885336
, 48110377
, 48416658
, 49698743
, 49890376
, 53789117
, 56422224
, 5663656
, 57298708
, 57322840
, 597352
, 7847724
, 80830904
, 8149566
, 8153417
, 855703
, 9969
|
|||||
| ChEBI ID |
CHEBI:9683
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [2] | |
| References | ||||||
| 1 | Trichloromethiazide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Prediction of the overall renal tubular secretion and hepatic clearance of anionic drugs and a renal drug-drug interaction involving organic anion transporter 3 in humans by in vitro uptake experiments. Drug Metab Dispos. 2011 Jun;39(6):1031-8. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
