Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00849
|
|||||
| Drug Name |
Lidocaine
|
|||||
| Synonyms |
2-(Diethylamino)-2',6'-acetoxylidide; 2-(Diethylamino)-N-(2,6-dimethylphenyl)acetamide; 2-Diethylamino-N-(2,6-dimethyl-phenyl)-acetamide; 2-Diethylamino-N-(2,6-dimethylphenyl)acetamide; After Burn Double Strength Gel; After Burn Double Strength Spray; After Burn Gel; After Burn Spray; Alfa-Dietilamino-2,6-dimetilacetanilide; Alfa-Dietilamino-2,6-dimetilacetanilide [Italian]; Alpha-(Diethylamino)-2,6-acetoxylidide; Alpha-Diethylamino-2,6-dimethylacetanilide; Alpha-Diethylaminoaceto-2,6-xylidide; Alphacaine; Anestacon; Anestacon Jelly; CDS1_000283; Cappicaine; Cito optadren; Cuivasil; Dalcaine; Dentipatch; Dentipatch (TN); DermaFlex; Diethylaminoaceto-2,6-xylidide; Dilocaine; Duncaine; ELA-Max; EMBOLEX; Emla Cream; Esracaine; Gravocain; Isicaina; Isicaine; Jetocaine; L-Caine; L1026_SIGMA; LIDOCAINE (73-58-6 (MONOHYDROCHLORIDE); LIDOPEN; LQZ; Lanabiotic; Leostesin; Lida-Mantle; Lidocaina; Lidocaina [INN-Spanish]; Lidocaine (JP15/USP/INN); Lidocaine (VAN); Lidocaine Carbonate; Lidocaine Hydrocarbonate; Lidocaine Monohydrochloride; Lidocaine [USAN:INN:JAN]; Lidocainum; Lidocainum [INN-Latin]; Lidocaton; Lidoderm; Lidoject-1; Lidoject-2; Lignocaine; Lignocainum; Lingocaine; Maricaine; N-(2,6-dimethylphenyl)-N(2),N(2)-diethylglycinamide; N-(2,6-dimethylphenyl)-N~2~,N~2~-diethylglycinamide; Norwood Sunburn Spray; Octocaine; Octocaine-100; Octocaine-50; Omega-Diethylamino-2,6-dimethylacetanilide; Remicaine; Rocephin Kit; Rucaina; Solarcaine aloe extraburn relief cream; Solcain; Xilina; Xilocaina; Xilocaina [Italian]; Xllina; Xycaine; Xylestesin; Xylesthesin; Xylocain; Xylocaine; Xylocaine (TN); Xylocaine 5% Spinal; Xylocaine CO2; Xylocaine Dental Ointment; Xylocaine Endotracheal; Xylocaine Test Dose; Xylocaine Viscous; Xylocaine-Mpf; Xylocaine-Mpf with Glucose; Xylocard; Xylocitin; Xyloneural (free base); Xylotox; Zilactin-L; Zingo
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Ventricular tachycardia [ICD11: BC71.0] | Approved | [1] | |||
| Therapeutic Class |
Anesthetics
|
|||||
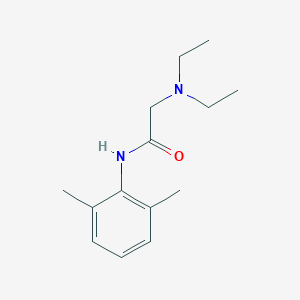
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C14H22N2O
|
|||||
| Canonical SMILES |
CCN(CC)CC(=O)NC1=C(C=CC=C1C)C
|
|||||
| InChI |
InChI=1S/C14H22N2O/c1-5-16(6-2)10-13(17)15-14-11(3)8-7-9-12(14)4/h7-9H,5-6,10H2,1-4H3,(H,15,17)
|
|||||
| InChIKey |
NNJVILVZKWQKPM-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 137-58-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 234.34 | Topological Polar Surface Area | 32.299 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
2.3
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
ChEBI:6456
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Lidocaine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
