Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00855
|
|||||
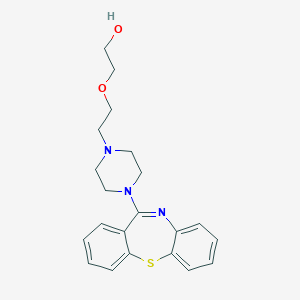
| Drug Name |
Quetiapine
|
|||||
| Synonyms |
111974-69-7; 2-(2-(4-Dibenzo(b,f)(1,4)thiazepin-11-yl-1-piperazinyl)ethoxy)ethanol; 2-[2-(4-Dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]ethanol; 2-[2-(4-dibenzo[b,f][1,4]thiazepin-11-ylpiperazin-1-yl)ethoxy]ethanol; 2-{[2-(4-dibenzo[b,f][1,4]thiazepin-11-ylpiperazin-1-yl)ethyl]oxy}ethanol; BGL0JSY5SI; C21H25N3O2S; CHEBI:8707; CHEMBL716; Co-Quetiapine; Ethanol, 2-[2-(4-dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]-; Ketipinor (TN); NCGC00095911-03; Norsic; Norsic (TN); PD-172760; Quetiapina; Quetiapine (INN); Quetiapine [INN:BAN]; Quetiapine hemifumarate; Quetiapinum; Seroquel (Fumarate); Seroquel (TN); UNII-BGL0JSY5SI; URKOMYMAXPYINW-UHFFFAOYSA-N; quetiapine
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Schizophrenia [ICD11: 6A20] | Approved | [1] | |||
| Therapeutic Class |
Antipsychotic Agents
|
|||||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C21H25N3O2S
|
|||||
| Canonical SMILES |
C1CN(CCN1CCOCCO)C2=NC3=CC=CC=C3SC4=CC=CC=C42
|
|||||
| InChI |
InChI=1S/C21H25N3O2S/c25-14-16-26-15-13-23-9-11-24(12-10-23)21-17-5-1-3-7-19(17)27-20-8-4-2-6-18(20)22-21/h1-8,25H,9-16H2
|
|||||
| InChIKey |
URKOMYMAXPYINW-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 111974-69-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 383.5 | Topological Polar Surface Area | 73.6 | ||
| Heavy Atom Count | 27 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
2.1
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
ChEBI:8707
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| SLC22A23 | Transporter Info | Solute carrier family 22 member 23 | Substrate | [3] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | P-GP | Transporter Info | Km = 12.3 microM | Baculovirus-infected insect cells-MDR1 | [4] | |
| References | ||||||
| 1 | Quetiapine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification of P-glycoprotein substrates and inhibitors among psychoactive compounds--implications for pharmacokinetics of selected substrates. J Pharm Pharmacol. 2004 Aug;56(8):967-75. | |||||
| 3 | Genome-wide association study of antipsychotic-induced QTc interval prolongation. Pharmacogenomics J. 2012 Apr;12(2):165-72. | |||||
| 4 | In vitro P-glycoprotein affinity for atypical and conventional antipsychotics. Life Sci. 2002 May 31;71(2):163-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
