Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00856
|
|||||
| Drug Name |
Mesalazine
|
|||||
| Synonyms |
2-Hydroxy-5-aminobenzoic acid; 3-carboxy-4-hydroxyaniline; 5 Aminosalicylate; 5 Aminosalicylic Acid; 5-AS; 5-ASA; 5-Amino-2-hydroxybenzoic acid; 5-Aminosalicylate; 5-Aminosalicylic acid; 5-amino-2-hydroxy-benzoic acid; 5-aminosalicylic acid, Mesalazine, Asacol, Pentasa, Canasa, Mesalamine; AJG-501; Allphar Brand of Mesalamine; Antigen Brand of Mesalamine; Apriso; Apriso (TN); Asacol; Asacol (TN); Asacol HD; Asacolitin; Asacolon; Asalit; Ascolitin; Axcan Brand of Mesalamine; Byk Brand of Mesalamine; Canasa; Canasa (TN); Celltech Brand of Mesalamine; Claversal; Falk Brand of Mesalamine; Farmasa Brand of Mesalamine; Ferring Brand of Mesalamine; Fisalamine; Fivasa; GlaxoSmithKline Brand of Mesalamine; Henning Berlin Brand of Mesalamine; Hydrochloride, Mesalamine; Iialda; Iialda (TN); Ipocal (TN); Ipocol; Lialda; Lialda (TN); Lixacol; M Aminosalicylic Acid; M-A; M-Aminosalicylic acid; MAX-002; MD-0901; Masacol (TN); Merckle Brand of Mesalamine; Mesacol; Mesalamine; Mesalamine (USP); Mesalamine Hydrochloride; Mesalamine Monosodium Salt; Mesalamine [USAN]; Mesalazina; Mesalazina[Spanish]; Mesalazine (JAN/INN); Mesalazine MMX; Mesalazinum; Mesalazinum [Latin]; Mesasal; Mesavance; Mesavancol; Meta Aminosalicylic Acid; Meta-AminosalicylicAcid; Mezavant; Mezavant XL; Minosalicylic acid; Monosodium Salt, Mesalamine; Norgine Brand of Mesalamine; Novo 5 ASA; Novo-5 ASA; Novo5 ASA; Novopharm Brand of Mesalamine; P-Aminosalicylsaeure; P-Aminosalicylsaeure [German]; Pentacol; Pentasa; Pentasa (TN); Procter & Gamble Brand of Mesalamine; Provalis Brand of Mesalamine; Rowasa; Rowasa (TN); SPD-476; SPD-480; Salicylic acid, 5-amino-(8CI); Salofalk; Salofalk (TN); Salofalk Granu-Stix; Salozinal; Sanofi Synthelabo Brand of Mesalamine; Schering Plough Brand of Mesalamine; Schering-Plough Brand of Mesalamine; SfRowasa; SmithKline Brand of Mesalamine; Solvay Brand of Mesalamine; Yamanouchi Brand of Mesalamine; Z-206
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Recurrent ventricular fibrillation [ICD11: BC71.1] | Approved | [1] | |||
| Therapeutic Class |
Antiinflammatory Agents
|
|||||
| Structure |
|
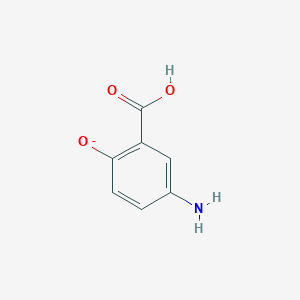 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C7H6NO3-
|
|||||
| Canonical SMILES |
C1=CC(=C(C=C1N)C(=O)O)[O-]
|
|||||
| InChI |
InChI=1S/C7H7NO3/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3,9H,8H2,(H,10,11)/p-1
|
|||||
| InChIKey |
KBOPZPXVLCULAV-UHFFFAOYSA-M
|
|||||
| CAS Number |
CAS 89-57-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 152.13 | Topological Polar Surface Area | 86.4 | ||
| Heavy Atom Count | 11 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
2
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
ChEBI:6775
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [2] | |
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [3] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [3] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [3] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OATP1B1 | Transporter Info | Km = 55.1 microM | Human embryonic kidney cells (HEK293)-OATP1B1 | [3] | |
| OATP1B3 | Transporter Info | Km = 77.4 microM | Human embryonic kidney cells (HEK293)-OATP1B3 | [3] | ||
| OATP2B1 | Transporter Info | Km = 188.9 microM | Human embryonic kidney cells (HEK293)-OATP2B1 | [3] | ||
| References | ||||||
| 1 | Mesalazine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Transport studies with 5-aminosalicylate. Eur J Clin Pharmacol. 2006 Oct;62(10):871-5. | |||||
| 3 | Role of organic anion-transporting polypeptides for cellular mesalazine (5-aminosalicylic acid) uptake. Drug Metab Dispos. 2011 Jun;39(6):1097-102. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
