Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00991
|
|||||
| Drug Name |
Lisinopril
|
|||||
| Synonyms |
(2S)-1-[(2S)-6-amino-2-[[(2S)-1-hydroxy-1-oxo-4-phenylbutan-2-yl]amino]hexanoyl]pyrrolidine-2-carboxylic acid; (S)-1-(N(2)-(1-carboxy-3-phenylpropyl)-L-lysyl)-L-proline; (S)-1-(N(sup 2)-(1-Carboxy-3-phenylpropyl)-L-lysyl)-L-proline; (S)-1-(N2-(1-Carboxy-3-phenylpropyl)-L-lysyl)-L-proline; 1-(N2-(1-Carboxy-3-phenylpropyl)-L-lysyl)-L-proline; 1-[Nalpha-[(S)-1-Carboxy-3-phenylpropyl]-L-lysyl]-L-proline; Acerbon; Acercomp; Alapril; Carace; Cipral; Cipril; Coric; Doneka; Hipril (TN); Inhibril; Inopril; LPR; Linopril; Linvas; Lipril; Lisinal; Lisinopril (INN); Lisinopril (anhydrous); Lisinopril anhydrous; Lisinoprilum; Lisinoprilum [Latin]; Lisipril; Lisoril; Lispril; Longes; Loril; Lysinopril; MK 521; MK 522; MK-521; N(2)-[(1S)-1-carboxy-3-phenylpropyl]-L-lysyl-L-proline; N-(1(S)-Carboxy-3-phenylpropyl)-L-lysyl-L-proline; N2-((S)-1-Carboxy-3-phenylpropyl)-L-lysyl-L-proline; N2-[(1S)-1-carboxy-3-phenylpropyl]-L-lysyl-L-proline; Noperten; Novatec; Presiten; Prinil; Prinivil; Prinivil (TN); Sinopril; Sinopryl; Tensopril; Tensopril (TN); Tensyn; Tersif; Vivatec; Zestril; Zestril (TN); [N2-[(S)-1-CARBOXY-3-PHENYLPROPYL]-L-LYSYL-L-PROLINE
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | High blood pressure [ICD11: BA00] | Approved | [1] | |||
| Therapeutic Class |
Antihypertensive Agents
|
|||||
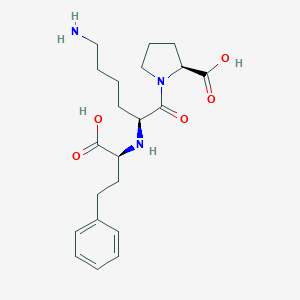
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C21H31N3O5
|
|||||
| Canonical SMILES |
C1CC(N(C1)C(=O)C(CCCCN)NC(CCC2=CC=CC=C2)C(=O)O)C(=O)O
|
|||||
| InChI |
InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1
|
|||||
| InChIKey |
RLAWWYSOJDYHDC-BZSNNMDCSA-N
|
|||||
| CAS Number |
CAS 76547-98-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 405.5 | Topological Polar Surface Area | 133 | ||
| Heavy Atom Count | 29 | Rotatable Bond Count | 12 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
-2.9
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:43755
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [2] | |
| References | ||||||
| 1 | Rosuvastatin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Peptide transporter substrate identification during permeability screening in drug discovery: comparison of transfected MDCK-hPepT1 cells to Caco-2 cells. Arch Pharm Res. 2007 Apr;30(4):507-18. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
