Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01170
|
|||||
| Drug Name |
Toremifene
|
|||||
| Synonyms |
(Z)-2-(4-(4-Chloro-1,2-diphenyl-1-butenyl)phenoxy)-N,N-dimethylethanamine; 2-(p-[(Z)-4-chloro-1,2-diphenyl-1-butenyl]-phenoxy)-N,N-dimethyl-ethylamine citrate(1:1); 2-(para-((Z)-4-Chloro-1,2-diphenyl-1-butenyl)phenoxy)-N,N-dimethylethylamine (IUPAC); 2-({4-[(1Z)-4-chloro-1,2-diphenylbut-1-en-1-yl]phenyl}oxy)-N,N-dimethylethanamine; 2-[4-[(Z)-4-chloro-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethylethanamine; 2-{4-[(1Z)-4-chloro-1,2-diphenylbut-1-en-1-yl]phenoxy}-N,N-dimethylethanamine; Acapodene; Acapodene (TN); Estrimex; FC-1157a; Fareston (TN); Farestone; GTX-006 (Acapodene); GTx 006; GTx-006; Toremifene (INN); Toremifene Base; Toremifene Citrate (1:1); Toremifene [INN:BAN]; Toremifeno; Toremifeno [Spanish]; Toremifenum; Toremifenum [Latin]; Z-Toremifene; {2-[4-(4-Chloro-1,2-diphenyl-but-1-enyl)-phenoxy]-ethyl}-dimethyl-amine
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Breast cancer [ICD11: 2C60-2C6Z] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
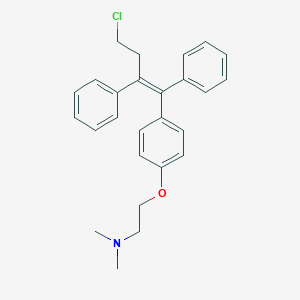
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C26H28ClNO
|
|||||
| Canonical SMILES |
CN(C)CCOC1=CC=C(C=C1)C(=C(CCCl)C2=CC=CC=C2)C3=CC=CC=C3
|
|||||
| InChI |
InChI=1S/C26H28ClNO/c1-28(2)19-20-29-24-15-13-23(14-16-24)26(22-11-7-4-8-12-22)25(17-18-27)21-9-5-3-6-10-21/h3-16H,17-20H2,1-2H3/b26-25-
|
|||||
| InChIKey |
XFCLJVABOIYOMF-QPLCGJKRSA-N
|
|||||
| CAS Number |
CAS 89778-26-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 406 | Topological Polar Surface Area | 12.5 | ||
| Heavy Atom Count | 29 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
7.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10038137
, 103628891
, 10366
, 111617870
, 11528602
, 117870220
, 12013627
, 124892293
, 126621304
, 126648750
, 126669839
, 127316634
, 127316635
, 127316636
, 127316637
, 127316638
, 127316639
, 127316640
, 127316641
, 127316642
, 127316643
, 127316644
, 127316645
, 127316646
, 127316647
, 127316648
, 127316649
, 127316650
, 127316651
, 127316652
, 127316653
, 127316654
, 127316655
, 14757386
, 29216214
, 36055011
, 46506087
, 48416648
, 53787422
, 56352877
, 57410179
, 76883860
, 7980819
, 92309038
, 92718822
, 93165674
, 93166369
, 93167220
, 93626357
, 96025304
|
|||||
| ChEBI ID |
ChEBI:9635
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Toremifene was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Antiestrogens and steroid hormones: substrates of the human P-glycoprotein. Biochem Pharmacol. 1994 Jul 19;48(2):287-92. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
