Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01177
|
|||||
| Drug Name |
Hydroxyurea
|
|||||
| Synonyms |
1-HYDROXYUREA; Biosupressin; Carbamide oxide; Carbamohydroxamic acid; Carbamohydroximic acid; Carbamohydroxyamic acid; Carbamoyl oxime; Carbamyl hydroxamate; Carrbamoyl Oxime; DRG-0253; Droxia; Droxia (TM); Droxia (TN); H 8627; HYDREA (TN); HYDROXY-UREA; Hidrix; Hidroxicarbamida; Hidroxicarbamida [INN-Spanish]; Hydrea; Hydrea (TM); Hydrea, Biosupressin, Cytodrox, Hydroxyurea; Hydreia; Hydroxicarbamidum; Hydroxyaminomethanamide; Hydroxycarbamid; Hydroxycarbamide; Hydroxycarbamide (JAN/INN); Hydroxycarbamidum; Hydroxycarbamidum [INN-Latin]; Hydroxycarbamine; Hydroxyharnstoff; Hydroxyharnstoff [German]; Hydroxylurea; Hydroxyurea (D4); Hydroxyurea (USP); Hydroxyurea [USAN:BAN]; Hydroxyurea(d4); Hydura; Hydurea; Idrossicarbamide; Idrossicarbamide [DCIT]; Litaler; Litalir; Mylocel; N-(Aminocarbonyl) Hydroxyamine; N-Carbamoylhydroxylamine; N-HYDROXY UREA; N-Hydroxymocovina; N-Hydroxymocovina [Czech]; N-Hydroxyurea; NHY; Onco-carbide; Oncocarbide; Oxyurea; S-phase/G-1 interface inhibitor; SK 22591; SQ 1089; SQ-1089; Siklos; Tetratogen: inhibits ribonucleoside diphosphate reductase
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Chronic myelogenous leukemia [ICD11: 2A20.0] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
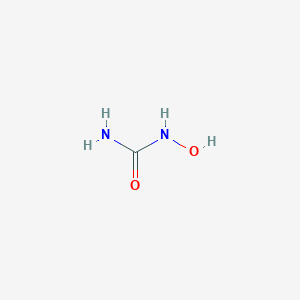
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
CH4N2O2
|
|||||
| Canonical SMILES |
C(=O)(N)NO
|
|||||
| InChI |
InChI=1S/CH4N2O2/c2-1(4)3-5/h5H,(H3,2,3,4)
|
|||||
| InChIKey |
VSNHCAURESNICA-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 127-07-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 76.055 | Topological Polar Surface Area | 75.4 | ||
| Heavy Atom Count | 5 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
-1.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
11111283
, 11120331
, 11120819
, 11121307
, 11335731
, 11360970
, 11363803
, 11366365
, 11368927
, 11371484
, 11373522
, 11377089
, 11404371
, 11407166
, 11446788
, 11461942
, 11483983
, 11487880
, 11490278
, 11491827
, 11494723
, 11538819
, 12014598
, 14747331
, 17137218
, 17405158
, 24278474
, 24879294
, 26611777
, 26679201
, 26697171
, 26747533
, 26747534
, 26747535
, 26758911
, 29222781
, 30388908
, 3135371
, 32810068
, 5137976
, 601956
, 7847407
, 7849615
, 7979551
, 8136972
, 8149381
, 8152311
, 842099
, 90752
, 9256
|
|||||
| ChEBI ID |
ChEBI:44423
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [2] | |
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [2] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [2] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Hydroxyurea was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Transcellular movement of hydroxyurea is mediated by specific solute carrier transporters. Exp Hematol. 2011 Apr;39(4):446-56. | |||||
| 3 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
