Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01194
|
|||||
| Drug Name |
Cefotiam
|
|||||
| Synonyms |
(6R,7R)-7-[2-(2-Amino-thiazol-4-yl)-acetylamino]-3-[1-(2-dimethylamino-ethyl)-1H-tetrazol-5-ylsulfanylmethyl]-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[2-(2-amino-1,3-thiazol-4-yl)acetyl]amino]-3-[[1-(2-dimethylaminoethyl)tetrazol-5-yl]sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2-amino-1,3-thiazol-4-yl)acetyl]amino}-3-[({1-[2-(dimethylamino)ethyl]-1H-tetrazol-5-yl}sulfanyl)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2-amino-1,3-thiazol-4-yl)acetyl]amino}-3-[({1-[2-(dimethylamino)ethyl]-1H-tetrazol-5-yl}thio)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7b-[2-(aminothiazol-4-yl)acetamido]-3-[[[1-(2-dimethylaminoethyl)-1h-tetrazol-5-yl]thio]methyl]ceph-3-em-4-carboxylic acid; 7beta-(2-amino-1,3-thiazol-4-yl)acetamido-3-[({1-[2-(dimethylamino)ethyl]-1H-tetrazol-5-yl}sulfanyl)methyl]-3,4-didehydrocepham-4-carboxylic acid; Abbott-48999; Aspil; Aspil (TN); CEFOTIAM HYDROCHLORIDE; CGP 14221E; CGP-14221-E; CTM; Cefotiam (INN); Cefotiam [INN:BAN]; Cefotiamum; Cefotiamum [INN-Latin]; Ceradolan; Haloapor; SCE-963
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Fungal infections [ICD11: 1F20-1F2Z] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
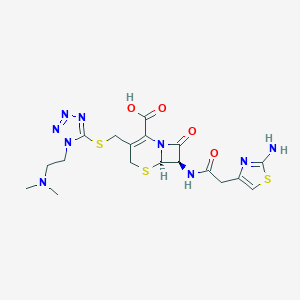 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C18H23N9O4S3
|
|||||
| Canonical SMILES |
CN(C)CCN1C(=NN=N1)SCC2=C(N3C(C(C3=O)NC(=O)CC4=CSC(=N4)N)SC2)C(=O)O
|
|||||
| InChI |
InChI=1S/C18H23N9O4S3/c1-25(2)3-4-26-18(22-23-24-26)34-7-9-6-32-15-12(14(29)27(15)13(9)16(30)31)21-11(28)5-10-8-33-17(19)20-10/h8,12,15H,3-7H2,1-2H3,(H2,19,20)(H,21,28)(H,30,31)/t12-,15-/m1/s1
|
|||||
| InChIKey |
QYQDKDWGWDOFFU-IUODEOHRSA-N
|
|||||
| CAS Number |
CAS 61622-34-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 525.6 | Topological Polar Surface Area | 251 | ||
| Heavy Atom Count | 34 | Rotatable Bond Count | 10 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 13 | |||
| XLogP |
-2.4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103401140
, 104343035
, 11466510
, 11467630
, 11486000
, 117600521
, 124766419
, 126670035
, 134224539
, 134338422
, 135006449
, 136054981
, 137006379
, 140253709
, 143261064
, 14763007
, 14836521
, 160963577
, 163693587
, 164788213
, 174549096
, 179150782
, 196394354
, 198976567
, 223676320
, 226515016
, 236431513
, 249865493
, 251912556
, 251916666
, 252073508
, 252358745
, 34709097
, 46506679
, 47662540
, 47811024
, 47885651
, 48334777
, 48415723
, 49699361
, 50050897
, 50124314
, 50998350
, 51091952
, 8178318
, 85547195
, 85788073
|
|||||
| ChEBI ID |
CHEBI:355510
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [2] | |
| References | ||||||
| 1 | Cefotiam was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Adaptive responses of renal organic anion transporter 3 (OAT3) during cholestasis. Am J Physiol Renal Physiol. 2008 Jul;295(1):F247-52. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
