Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01200
|
|||||
| Drug Name |
Meropenem
|
|||||
| Synonyms |
(1R,5S,6S)-2-[(3S,5S)-5-(dimethylaminocarbonyl)pyrrolidin-3-ylthio]-6-[(R)-1-hydroxyethyl]-1-methylcarbapen-2-em-3-carboxylic acid trihydrate; (2S,3R,4R)-2-[(1S,2R)-1-carboxy-2-hydroxypropyl]-4-{[(3S,5R)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl}-3-methyl-3,4-dihydro-2H-pyrrole-5-carboxylic acid; (4R,5S,6S)-3-[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (4R,5S,6S)-3-{[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]thio}-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (5S,6S)-3-((3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-ylthio)-6-((S)-1-hydroxyethyl)-4-methyl-7-oxo-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (6S)-2-{[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl}-6-[(1R)-1-hydroxyethyl]-1beta-methyl-2,3-didehydro-1-carbapenam-3-carboxylic acid; MEPM; MERONEM; Mepem (TN); Meronem; Meronem (TN); Meropen; Meropen (TN); Meropenem (INN); Meropenem anhydrous; Merrem; Merrem (TN); Merrem I.V. (TN); Neopenem (TN); SM-7338
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Meningitis [ICD11: 1D01.Y] | Approved | [1] | |||
| Pneumonia [ICD11: CA40] | Approved | [1] | ||||
| Sepsis [ICD11: 1G40-1G41] | Approved | [1] | ||||
| Anthrax [ICD11: 1B97] | Approved | [1] | ||||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
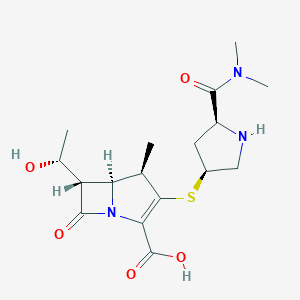 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C17H25N3O5S
|
|||||
| Canonical SMILES |
CC1C2C(C(=O)N2C(=C1SC3CC(NC3)C(=O)N(C)C)C(=O)O)C(C)O
|
|||||
| InChI |
InChI=1S/C17H25N3O5S/c1-7-12-11(8(2)21)16(23)20(12)13(17(24)25)14(7)26-9-5-10(18-6-9)15(22)19(3)4/h7-12,18,21H,5-6H2,1-4H3,(H,24,25)/t7-,8-,9+,10+,11-,12-/m1/s1
|
|||||
| InChIKey |
DMJNNHOOLUXYBV-PQTSNVLCSA-N
|
|||||
| CAS Number |
CAS 119478-56-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 383.5 | Topological Polar Surface Area | 136 | ||
| Heavy Atom Count | 26 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
-2.4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10298746
, 103188146
, 103914625
, 104624717
, 11112878
, 11467134
, 11468254
, 11486822
, 117664409
, 117695442
, 119525880
, 12014124
, 121363729
, 124658855
, 124766033
, 126592940
, 127755594
, 134221905
, 135022991
, 135684925
, 135685918
, 135692126
, 135693793
, 135727273
, 136375187
, 137003432
, 137179129
, 139117093
, 14780292
, 14902620
, 26744414
, 36885035
, 46386666
, 46504928
, 47217060
, 47440550
, 47589234
, 47811032
, 49681817
, 49699298
, 50123967
, 57403544
, 78847646
, 7979907
, 92126058
, 92308358
, 92309041
, 93167009
, 96024875
, 98569498
|
|||||
| ChEBI ID |
ChEBI:6770
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [2] | |
| References | ||||||
| 1 | Meropenem was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Characterization of CS-023 (RO4908463), a novel parenteral carbapenem antibiotic, and meropenem as substrates of human renal transporters. Drug Metab Pharmacokinet. 2007 Feb 25;22(1):41-7. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
