Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01235
|
|||||
| Drug Name |
Celiprolol
|
|||||
| Synonyms |
3-[3-acetyl-4-[3-(tert-butylamino)-2-hydroxypropoxy]phenyl]-1,1-diethylurea; BRN 2776298; CCRIS 3400; Celectol; Celiprolol [INN:BAN]; Celiprololum; Celiprololum [INN-Latin]; EINECS 260-497-7; N'-(3-Acetyl-4-(3-((1,1-dimethylethyl)amino)-2-hydroxypropoxy)phenyl)-N,N-diethylurea; RHC 5320 A; RHC-5320A; ST-1396; Selectol; UL/1677; Urea, N'-(3-acetyl-4-(3-((1,1-dimethylethyl)amino)-2-hydroxypropoxy)phenyl)-N,N-diethyl-; celiprolol
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | High blood pressure [ICD11: BA00] | Approved | [1] | |||
| Ehlers-Danlos syndrome [ICD11: LD28.1] | Approved | [1] | ||||
| Huntington disease [ICD11: 8A01.10] | Preclinical | [1] | ||||
| Therapeutic Class |
Beta Blockers
|
|||||
| Structure |
|
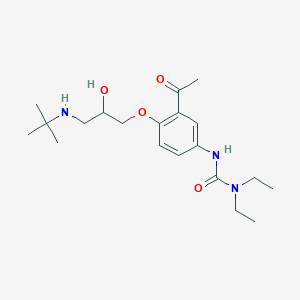 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C20H33N3O4
|
|||||
| Canonical SMILES |
CCN(CC)C(=O)NC1=CC(=C(C=C1)OCC(CNC(C)(C)C)O)C(=O)C
|
|||||
| InChI |
InChI=1S/C20H33N3O4/c1-7-23(8-2)19(26)22-15-9-10-18(17(11-15)14(3)24)27-13-16(25)12-21-20(4,5)6/h9-11,16,21,25H,7-8,12-13H2,1-6H3,(H,22,26)
|
|||||
| InChIKey |
JOATXPAWOHTVSZ-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 56980-93-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 379.5 | Topological Polar Surface Area | 90.9 | ||
| Heavy Atom Count | 27 | Rotatable Bond Count | 10 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
1.9
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:94461
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Analogue-based Drug Discovery | |||||
| 2 | Involvement of influx and efflux transport systems in gastrointestinal absorption of celiprolol. J Pharm Sci. 2009 Jul;98(7):2529-39. | |||||
| 3 | Relationship between urinary sodium excretion and pioglitazone-induced edema. J Diabetes Investig. 2010 Oct 19;1(5):208-11. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
