Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01261
|
|||||
| Drug Name |
Nadolol
|
|||||
| Synonyms |
(2R,3S)-5-(3-(tert-Butylamino)-2-hydroxypropoxy)-1,2,3,4-tetrahydronaphthalene-2,3-diol; (2R,3S)-5-({3-[(1,1-dimethylethyl)amino]-2-hydroxypropyl}oxy)-1,2,3,4-tetrahydronaphthalene-2,3-diol; (2R,3S)-5-[3-(tert-butylamino)-2-hydroxypropoxy]-1,2,3,4-tetrahydronaphthalene-2,3-diol; 2,3-cis-1,2,3,4-Tetrahydro-5-((2-hydroxy-3-tert-butylamino)propoxy)-2,3-naphthalenediol; 5-(3-((1,1-Dimethylethyl)amino)-2-hydroxypropoxy)-1,2,3,4-tetrahydro-2,3-naphthalenediol; Alti-Nadolol (TN); Anabet; Anabet (TN); Apo-Nadol (TN); Corgard; Corgard (TN); Corgaretic; Corzide (TN); Nadic; Nadolol (JP15/USP/INN); Nadolol [USAN:BAN:INN:JAN]; Nadololum; Nadololum [INN-Latin]; Novo-Nadolol (TN); SQ 11725; SQ-11725; SQ11725; Solgol; Solgol (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Angina [ICD11: BA40] | Approved | [1] | |||
| High blood pressure [ICD11: BA00] | Approved | [1] | ||||
| Therapeutic Class |
Antihypertensive Agents
|
|||||
| Structure |
|
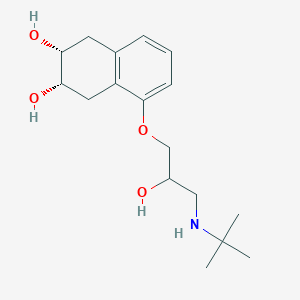 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C17H27NO4
|
|||||
| Canonical SMILES |
CC(C)(C)NCC(COC1=CC=CC2=C1CC(C(C2)O)O)O
|
|||||
| InChI |
InChI=1S/C17H27NO4/c1-17(2,3)18-9-12(19)10-22-16-6-4-5-11-7-14(20)15(21)8-13(11)16/h4-6,12,14-15,18-21H,7-10H2,1-3H3/t12?,14-,15+/m1/s1
|
|||||
| InChIKey |
VWPOSFSPZNDTMJ-UCWKZMIHSA-N
|
|||||
| CAS Number |
CAS 42200-33-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 309.4 | Topological Polar Surface Area | 82 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
0.7
|
|||||
| PubChem CID | ||||||
| PubChem SID |
855594
, 7847498
, 7980059
, 8149963
, 8175898
, 10321209
, 11335602
, 11360841
, 11364537
, 11367099
, 11369661
, 11372751
, 11373904
, 11377823
, 11461813
, 11466846
, 11467966
, 11485128
, 11486372
, 11489362
, 11491401
, 11492008
, 11495457
, 12013396
, 26612453
, 26680146
, 34705082
, 46505509
, 47216720
, 47440192
, 47515259
, 48035045
, 48110397
, 48416301
, 49995587
, 50105273
, 53787811
, 56413054
, 56422422
, 57312272
, 75643004
, 85787107
, 92124849
, 92125681
, 92307845
, 99301515
, 99351138
, 103189209
, 103913689
, 104169962
|
|||||
| ChEBI ID |
ChEBI:7444
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Nadolol was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Involvement of influx and efflux transport systems in gastrointestinal absorption of celiprolol. J Pharm Sci. 2009 Jul;98(7):2529-39. | |||||
| 3 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
