Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01264
|
|||||
| Drug Name |
Apremilast
|
|||||
| Synonyms |
Apremilast (USAN); CC-10004; N-[2-[1-(3-ethoxy-4-methoxy-phenyl)-2-methylsulfonyl-ethyl]-1,3-dioxo-isoindol-4-yl]acetamide
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Psoriatic arthritis [ICD11: FA21] | Approved | [1] | |||
| Plaque psoriasis [ICD11: EA90.0] | Approved | [1] | ||||
| Structure |
|
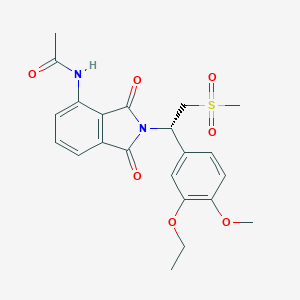 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C22H24N2O7S
|
|||||
| Canonical SMILES |
CCOC1=C(C=CC(=C1)C(CS(=O)(=O)C)N2C(=O)C3=C(C2=O)C(=CC=C3)NC(=O)C)OC
|
|||||
| InChI |
InChI=1S/C22H24N2O7S/c1-5-31-19-11-14(9-10-18(19)30-3)17(12-32(4,28)29)24-21(26)15-7-6-8-16(23-13(2)25)20(15)22(24)27/h6-11,17H,5,12H2,1-4H3,(H,23,25)/t17-/m1/s1
|
|||||
| InChIKey |
IMOZEMNVLZVGJZ-QGZVFWFLSA-N
|
|||||
| CAS Number |
CAS 608141-41-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 460.5 | Topological Polar Surface Area | 128 | ||
| Heavy Atom Count | 32 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
1.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
16663888
, 28672022
, 42703565
, 50086920
, 78553422
, 96025543
, 103594774
, 104103394
, 135195808
, 135299279
, 136946493
, 140792549
, 144115618
, 152256090
, 152258393
, 160647230
, 164045129
, 164194075
, 176253765
, 178103944
, 186014493
, 187575333
, 189561506
, 198938842
, 198994002
, 218902332
, 223380767
, 223443866
, 223593318
, 224603101
, 226643371
, 247101845
, 249492055
, 249814467
, 250163167
, 251962967
, 251970550
, 252088578
, 252160602
, 252214744
, 252438251
, 252451837
, 252553654
|
|||||
| ChEBI ID |
CHEBI:78540
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Apremilast was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
