Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01270
|
|||||
| Drug Name |
Ibrutinib
|
|||||
| Synonyms |
Ibrutinib (BTK inhibitor); PCI-32765
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Chronic lymphocytic leukemia [ICD11: 2A82.0] | Approved | [1] | |||
| Mantle cell lymphoma [ICD11: 2A85.5] | Approved | [1] | ||||
| Waldenstrom's macroglobulinemia [ICD11: 2A85.4] | Approved | [1] | ||||
| Structure |
|
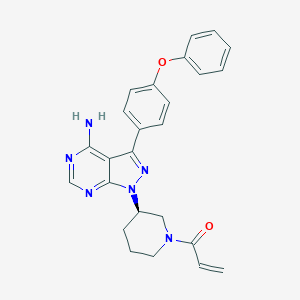 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C25H24N6O2
|
|||||
| Canonical SMILES |
C=CC(=O)N1CCCC(C1)N2C3=NC=NC(=C3C(=N2)C4=CC=C(C=C4)OC5=CC=CC=C5)N
|
|||||
| InChI |
InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1
|
|||||
| InChIKey |
XYFPWWZEPKGCCK-GOSISDBHSA-N
|
|||||
| CAS Number |
CAS 936563-96-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 440.5 | Topological Polar Surface Area | 99.2 | ||
| Heavy Atom Count | 33 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
3.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
49837088
, 57132050
, 123051065
, 124898784
, 124898785
, 136940589
, 136961336
, 137472671
, 152258295
, 160647134
, 162202692
, 163312254
, 163679284
, 164045821
, 164193915
, 165245631
, 171572071
, 172919350
, 174007139
, 175267389
, 176250273
, 178103494
, 184611713
, 184816969
, 189025882
, 198993428
, 215784879
, 223375297
, 223485070
, 223600477
, 223685684
, 223704724
, 224184328
, 226558356
, 242585660
, 247523488
, 249736825
, 251971042
, 252088605
, 252110183
, 252160516
, 252215999
, 252451849
|
|||||
| ChEBI ID |
CHEBI:76612
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Ibrutinib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | P-Glycoprotein (MDR1/ABCB1) Restricts Brain Penetration of the Bruton's Tyrosine Kinase Inhibitor Ibrutinib, While Cytochrome P450-3A (CYP3A) Limits Its Oral Bioavailability. Mol Pharm. 2018 Nov 5;15(11):5124-5134. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
