Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01274
|
|||||
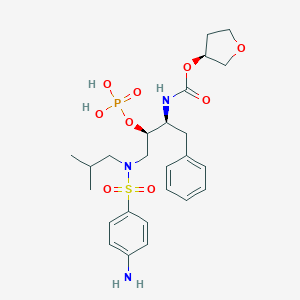
| Drug Name |
Fosamprenavir
|
|||||
| Synonyms |
((3S)Oxolan-3-yloxy)-N-((1S,2R)-3-{[(4-aminophenyl)sulfonyl](2-methylpropyl)amino}-1-benzyl-2-(phosphonooxy)propyl)carboxamide; (3-(((4-Aminophenyl)sulfonyl)(2-methylpropyl)amino)-1-(phenylmethyl)-2-(phosphonooxy)propyl)carbamic acid C-(tetrahydro-3-furanyl) ester; Amprenavir phosphate; Carbamic acid, ((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-1-(phenylmethyl)-2-(phosphonooxy)propyl)-, C-((3S)-tetrahydro-3-furanyl) ester; Fosamprenavir (INN); Fosamprenavir [INN]; GW 433908; GW433908; GW433908A (*Sodium Salt*); GW433908G (*Calcium Salt*); Lexiva (TM); Lexiva (TN); Telzir; Telzir (TN); Telzir(TM); VX 175; VX-175; [(3S)-oxolan-3-yl] N-[(2S,3R)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-1-phenyl-3-phosphonooxybutan-2-yl]carbamate
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Human immunodeficiency virus infection [ICD11: 1C62.Z] | Approved | [1] | |||
| Therapeutic Class |
Anti-HIV Agents
|
|||||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C25H36N3O9PS
|
|||||
| Canonical SMILES |
CC(C)CN(CC(C(CC1=CC=CC=C1)NC(=O)OC2CCOC2)OP(=O)(O)O)S(=O)(=O)C3=CC=C(C=C3)N
|
|||||
| InChI |
InChI=1S/C25H36N3O9PS/c1-18(2)15-28(39(33,34)22-10-8-20(26)9-11-22)16-24(37-38(30,31)32)23(14-19-6-4-3-5-7-19)27-25(29)36-21-12-13-35-17-21/h3-11,18,21,23-24H,12-17,26H2,1-2H3,(H,27,29)(H2,30,31,32)/t21-,23-,24+/m0/s1
|
|||||
| InChIKey |
MLBVMOWEQCZNCC-OEMFJLHTSA-N
|
|||||
| CAS Number |
CAS 226700-79-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 585.6 | Topological Polar Surface Area | 186 | ||
| Heavy Atom Count | 39 | Rotatable Bond Count | 14 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 11 | |||
| XLogP |
1.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
630866
, 10242897
, 14764609
, 14911487
, 17396671
, 29309884
, 46504901
, 50070552
, 50071323
, 75335910
, 103633149
, 104375333
, 126680953
, 134222910
, 134337996
, 135111213
, 136139133
, 137006000
, 142147341
, 160964617
, 175267520
, 179296052
, 210279023
, 223447627
, 223664897
, 226420602
, 251971352
|
|||||
| ChEBI ID |
CHEBI:82941
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Fosamprenavir was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
