Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01289
|
|||||
| Drug Name |
Dasabuvir
|
|||||
| Synonyms |
1132935-63-7; ABT 333; ABT-333; ABT333; C26H27N3O5S; CHEBI:85182; DE54EQW8T1; Dasabuvir (ABT-333); Dasabuvir (USA; Dasabuvir [INN]; Dasabuvir [USAN:INN]; N-(6-(3-(tert-butyl)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-methoxyphenyl)naphthalen-2-yl)methanesulfonamide; N-(6-(3-tert-Butyl-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-methoxyphenyl)naphthalen-2-yl)methanesulfonamide; N-{6-[3-tert-butyl-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-methoxyphenyl]naphthalen-2-yl}methanesulfonamide; UNII-DE54EQW8T1
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Hepatitis C virus infection [ICD11: 1E50.2, 1E51.1] | Phase 3 | [1] | |||
| Structure |
|
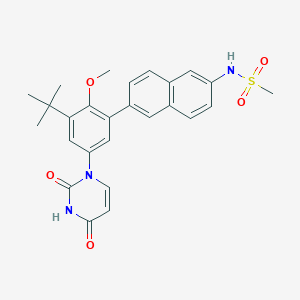 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C26H27N3O5S
|
|||||
| Canonical SMILES |
CC(C)(C)C1=CC(=CC(=C1OC)C2=CC3=C(C=C2)C=C(C=C3)NS(=O)(=O)C)N4C=CC(=O)NC4=O
|
|||||
| InChI |
InChI=1S/C26H27N3O5S/c1-26(2,3)22-15-20(29-11-10-23(30)27-25(29)31)14-21(24(22)34-4)18-7-6-17-13-19(28-35(5,32)33)9-8-16(17)12-18/h6-15,28H,1-5H3,(H,27,30,31)
|
|||||
| InChIKey |
NBRBXGKOEOGLOI-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 1132935-63-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 493.6 | Topological Polar Surface Area | 113 | ||
| Heavy Atom Count | 35 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
4.3
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:85182
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | ClinicalTrials.gov (NCT02582632) A Study to Evaluate Ombitasvir/Paritaprevir/Ritonavir and Dasabuvir in Treatment-Nave Hepatitis C Virus Genotype 1b-Infected Adults | |||||
| 2 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
