Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01290
|
|||||
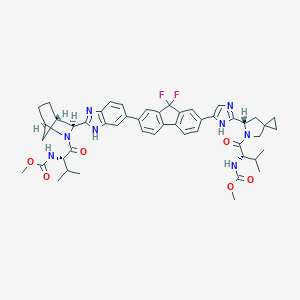
| Drug Name |
Ledipasvir
|
|||||
| Synonyms |
013TE6E4WV; 1256388-51-8; CHEBI:85089; CHEMBL2374220; DTXSID90154829; EX-A411; GS 5885; GS-5885; GS5885; Ledipasvir (GS5885); Ledipasvir (USAN); Ledipasvir [USAN:INN]; MolPort-039-138-665; SCHEMBL15116943; SCHEMBL2706494; UNII-013TE6E4WV; WHO 9796; methyl [(2S)-1-{(6S)-6-[4-(9,9-difluoro-7-{2-[(1R,3S,4S)-2-{(2S)-2-[(methoxycarbonyl)amino]-3-methylbutanoyl}-2-azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-5-yl}-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-azaspiro[2.4]hept-5-yl}-3-methyl-1-oxobutan-2-yl]carbamate
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Hepatitis C virus infection [ICD11: 1E50.2, 1E51.1] | Approved | [1] | |||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C49H54F2N8O6
|
|||||
| Canonical SMILES |
CC(C)C(C(=O)N1CC2(CC2)CC1C3=NC=C(N3)C4=CC5=C(C=C4)C6=C(C5(F)F)C=C(C=C6)C7=CC8=C(C=C7)N=C(N8)C9C1CCC(C1)N9C(=O)C(C(C)C)NC(=O)OC)NC(=O)OC
|
|||||
| InChI |
InChI=1S/C49H54F2N8O6/c1-24(2)39(56-46(62)64-5)44(60)58-23-48(15-16-48)21-38(58)42-52-22-37(55-42)28-9-13-32-31-12-8-26(18-33(31)49(50,51)34(32)19-28)27-10-14-35-36(20-27)54-43(53-35)41-29-7-11-30(17-29)59(41)45(61)40(25(3)4)57-47(63)65-6/h8-10,12-14,18-20,22,24-25,29-30,38-41H,7,11,15-17,21,23H2,1-6H3,(H,52,55)(H,53,54)(H,56,62)(H,57,63)/t29-,30+,38-,39-,40-,41-/m0/s1
|
|||||
| InChIKey |
VRTWBAAJJOHBQU-KMWAZVGDSA-N
|
|||||
| CAS Number |
CAS 1256388-51-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 889 | Topological Polar Surface Area | 175 | ||
| Heavy Atom Count | 65 | Rotatable Bond Count | 12 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 10 | |||
| XLogP |
7.4
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:85089
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Ledipasvir was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
