Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01299
|
|||||
| Drug Name |
Sunitinib malate
|
|||||
| Synonyms |
(Z)-N-(2-(diethylamino)ethyl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide (S)-2-hydroxysuccinate; LVX8N1UT73; MFCD08282795; N-(2-(Diethylamino)ethyl)-5-((Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide (2S)-hydroxybutanedioate; PHA-290940AD; SU 011248; SU010398; SU011248; SU011248 L-malate salt; SUNITINIB MALEATE; Sunitinib malate; Sunitinib malate [USAN]; UNII-LVX8N1UT73
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Renal cell carcinoma [ICD11: 2C90] | Approved | [1] | |||
| Gastrointestinal stromal tumor [ICD11: 2B5B] | Approved | [1] | ||||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
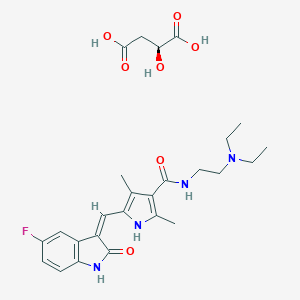 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C26H33FN4O7
|
|||||
| Canonical SMILES |
CCN(CC)CCNC(=O)C1=C(NC(=C1C)C=C2C3=C(C=CC(=C3)F)NC2=O)C.C(C(C(=O)O)O)C(=O)O
|
|||||
| InChI |
InChI=1S/C22H27FN4O2.C4H6O5/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28;5-2(4(8)9)1-3(6)7/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28);2,5H,1H2,(H,6,7)(H,8,9)/b17-12-;/t;2-/m.0/s1
|
|||||
| InChIKey |
LBWFXVZLPYTWQI-IPOVEDGCSA-N
|
|||||
| CAS Number |
CAS 341031-54-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 532.6 | Topological Polar Surface Area | 172 | ||
| Heavy Atom Count | 38 | Rotatable Bond Count | 10 | |||
| Hydrogen Bond Donor Count | 6 | Hydrogen Bond Acceptor Count | 9 | |||
| PubChem CID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Sunitinib malate was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | JNK-AKT-NF-kB controls P-glycoprotein expression to attenuate the cytotoxicity of deoxynivalenol in mammalian cells. Biochem Pharmacol. 2018 Oct;156:120-134. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
