Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01308
|
|||||
| Drug Name |
Glecaprevir
|
|||||
| Synonyms |
A-1282576.0; ABT 493; ABT-493; ABT-493(Glecaprevir); CS-8098; D10814; DB13879; EX-A1940; Glecaprevir; Glecaprevir (USAN/INN); Glecaprevir [USAN]; HY-17634; J3.646.120I; K6BUU8J72P; SB19760; SCHEMBL883097; UNII-K6BUU8J72P; glecaprevirum
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Chronic hepatitis C infection [ICD11: 1E51.1] | Phase 2 | [1] | |||
| Therapeutic Class |
Antiviral Agent
|
|||||
| Structure |
|
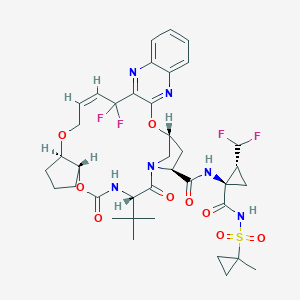 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C38H46F4N6O9S
|
|||||
| Canonical SMILES |
CC1(CC1)S(=O)(=O)NC(=O)C2(CC2C(F)F)NC(=O)C3CC4CN3C(=O)C(NC(=O)OC5CCCC5OCC=CC(C6=NC7=CC=CC=C7N=C6O4)(F)F)C(C)(C)C
|
|||||
| InChI |
InChI=1S/C38H46F4N6O9S/c1-35(2,3)28-32(50)48-19-20(17-24(48)30(49)46-37(18-21(37)29(39)40)33(51)47-58(53,54)36(4)14-15-36)56-31-27(43-22-9-5-6-10-23(22)44-31)38(41,42)13-8-16-55-25-11-7-12-26(25)57-34(52)45-28/h5-6,8-10,13,20-21,24-26,28-29H,7,11-12,14-19H2,1-4H3,(H,45,52)(H,46,49)(H,47,51)/b13-8+/t20-,21+,24+,25-,26-,28-,37-/m1/s1
|
|||||
| InChIKey |
MLSQGNCUYAMAHD-ITNVBOSISA-N
|
|||||
| CAS Number |
CAS 1365970-03-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 838.9 | Topological Polar Surface Area | 204 | ||
| Heavy Atom Count | 58 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 15 | |||
| XLogP |
4.6
|
|||||
| PubChem CID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | ClinicalTrials.gov (NCT02243280) A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Co-administration of ABT-493 and ABT-530 With and Without Ribavirin in Subjects With HCV Genotype 1, 4, 5, and 6 Infection | |||||
| 2 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
