Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01329
|
|||||
| Drug Name |
Auranofin
|
|||||
| Synonyms |
AURANOFIN; (1-Thio-beta-D-glucopyranosato)(triethylphosphine)gold 2,3,4,6-tetraacetate; CTK8F7877; KS-00001FB9; MMV688978; AKOS026750078; FT-0662343
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Rheumatoid arthritis [ICD11: FA20] | Approved | [1] | |||
| Therapeutic Class |
Antirheumatic Agents
|
|||||
| Structure |
|
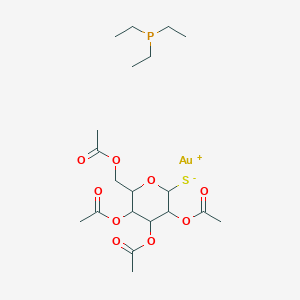 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C20H34AuO9PS
|
|||||
| Canonical SMILES |
CCP(CC)CC.CC(=O)OCC1C(C(C(C(O1)[S-])OC(=O)C)OC(=O)C)OC(=O)C.[Au+]
|
|||||
| InChI |
InChI=1S/C14H20O9S.C6H15P.Au/c1-6(15)19-5-10-11(20-7(2)16)12(21-8(3)17)13(14(24)23-10)22-9(4)18;1-4-7(5-2)6-3;/h10-14,24H,5H2,1-4H3;4-6H2,1-3H3;/q;;+1/p-1
|
|||||
| InChIKey |
AUJRCFUBUPVWSZ-UHFFFAOYSA-M
|
|||||
| CAS Number |
CAS 34031-32-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 678.5 | Topological Polar Surface Area | 115 | ||
| Heavy Atom Count | 32 | Rotatable Bond Count | 12 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 10 | |||
| PubChem CID | ||||||
| PubChem SID | ||||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MATE1 | Transporter Info | Multidrug and toxin extrusion protein 1 | Substrate | [2] | |
| OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [2] | ||
| References | ||||||
| 1 | Auranofin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Selection and characterization of a human ovarian cancer cell line resistant to auranofin. Oncotarget. 2017 Oct 9;8(56):96062-96078. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
