Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01343
|
|||||
| Drug Name |
Vinorelbine
|
|||||
| Synonyms |
Eunades; Exelbine; NVB; Navelbine; Vinorelbin; Vinorelbina; Vinorelbinum; Navelbine base; Vinorelbina [Spanish]; Vinorelbine Ditartarate; Vinorelbine ditartrate; Vinorelbine tartrate; Vinorelbinum [Latin]; KW 2307; KW 2307 base; ANX-530; KW-2307; Navelbine (TN); SDP-012; Vinorelbine (INN); Vinorelbine [INN:BAN]; Aspidospermidine-3-carboxylic acid; Nor-5'-anhydrovinblastine; Methyl (2beta,3beta,4beta,5alpha,12beta,19alpha)-4-acetoxy-15-[(6R,8S)-4-ethyl-8-(methoxycarbonyl)-1,3,6,7,8,9-hexahydro-2,6-methanoazecino[4,3-b]indol-8-yl]-3-hydroxy-16-methoxy-1-methyl-6,7-didehydroaspidospermidine-3-carboxylate; Methyl (2b,3b,4b,5a,12b,19a)-4-(acetyloxy)-15-[(6R,8S)-4-ethyl-8-(methoxycarbonyl)-1,3,6,7,8,9-hexahydro-2,6-methanoazecino[4,3-b]indol-8-yl]-3-hydroxy-16-methoxy-1-methyl-6,7-didehydroaspidospermidine-3-carboxylate
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Non-small cell lung cancer [ICD11: 2C25] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
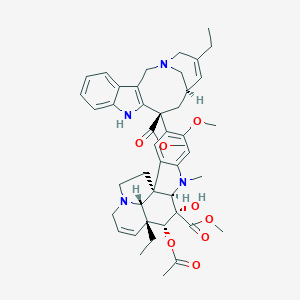
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C45H54N4O8
|
|||||
| Canonical SMILES |
CCC1=CC2CC(C3=C(CN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC
|
|||||
| InChI |
InChI=1S/C45H54N4O8/c1-8-27-19-28-22-44(40(51)55-6,36-30(25-48(23-27)24-28)29-13-10-11-14-33(29)46-36)32-20-31-34(21-35(32)54-5)47(4)38-43(31)16-18-49-17-12-15-42(9-2,37(43)49)39(57-26(3)50)45(38,53)41(52)56-7/h10-15,19-21,28,37-39,46,53H,8-9,16-18,22-25H2,1-7H3/t28-,37-,38+,39+,42+,43+,44-,45-/m0/s1
|
|||||
| InChIKey |
GBABOYUKABKIAF-IELIFDKJSA-N
|
|||||
| CAS Number |
CAS 71486-22-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 778.9 | Topological Polar Surface Area | 134 | ||
| Heavy Atom Count | 57 | Rotatable Bond Count | 10 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 11 | |||
| XLogP |
3.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
7980885
, 11056542
, 12013497
, 16335940
, 39341162
, 56311577
, 56312628
, 57288872
, 57359504
, 78753678
, 85670095
, 92309089
, 96025363
, 99031250
, 119526325
, 124893200
, 135049333
, 136123131
, 137001869
, 152258373
, 152344178
, 160647210
, 162011810
, 162178830
, 162224736
, 163312394
, 164831120
, 175266041
, 175608041
, 176484268
, 178103682
, 179116945
, 203356012
, 226396694
, 241376439
, 252157261
, 252359089
|
|||||
| ChEBI ID |
CHEBI:480999
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Vinorelbine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001 Nov;299(2):620-8. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
