Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01365
|
|||||
| Drug Name |
ABT-263
|
|||||
| Synonyms |
Navitoclax; ABT 263; S1001_Selleck; ABT263, Navitoclax; 4-(4-{[2-(4-chlorophenyl)-5,5-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({4-({(1R)-3-morpholin-4-yl-1-[(phenylsulfanyl)methyl]propyl}amino)-3-[(trifluoromethyl)sulfonyl]phenyl}sulfonyl)benzamide
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Myelofibrosis [ICD11: 2A20.2] | Phase 2 | [1] | |||
| Structure |
|
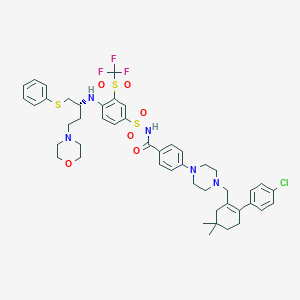 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C47H55ClF3N5O6S3
|
|||||
| Canonical SMILES |
CC1(CCC(=C(C1)CN2CCN(CC2)C3=CC=C(C=C3)C(=O)NS(=O)(=O)C4=CC(=C(C=C4)NC(CCN5CCOCC5)CSC6=CC=CC=C6)S(=O)(=O)C(F)(F)F)C7=CC=C(C=C7)Cl)C
|
|||||
| InChI |
InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1
|
|||||
| InChIKey |
JLYAXFNOILIKPP-KXQOOQHDSA-N
|
|||||
| CAS Number |
CAS 119229-65-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 974.6 | Topological Polar Surface Area | 170 | ||
| Heavy Atom Count | 65 | Rotatable Bond Count | 16 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 14 | |||
| XLogP |
9.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
56311703
, 56314453
, 57304426
, 57504587
, 87219189
, 87457108
, 96099448
, 99003698
, 99245529
, 99246089
, 99431767
, 99460835
, 103640740
, 104115920
, 124756924
, 124899202
, 124899203
, 125163731
, 126583646
, 126667000
, 126724167
, 131465096
, 131465716
, 134221945
, 135263748
, 135626669
, 135727392
, 136920253
, 137126833
, 141663047
, 143499177
, 152035716
, 152164573
, 152240012
, 152258081
, 152344157
, 160646920
, 162011689
, 162037376
, 162202714
, 163123180
, 163821637
, 164194132
, 164831796
, 174007035
, 174531509
, 198955115
, 204380851
, 223388517
, 223685382
|
|||||
| ChEBI ID |
CHEBI:94128
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | ClinicalTrials.gov (NCT03222609) A Study Evaluating Tolerability and Efficacy of Navitoclax in Combination With Ruxolitinib in Subjects With Myelofibrosis | |||||
| 2 | The B-cell lymphoma 2 (BCL2)-inhibitors, ABT-737 and ABT-263, are substrates for P-glycoprotein. Biochem Biophys Res Commun. 2011 May 6;408(2):344-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
