Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01378
|
|||||
| Drug Name |
99mTc-sestamibi
|
|||||
| Synonyms |
Sestamibi; RP-30; Tc-MIBI; Technetium-99m sestamibi
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Breast cancer [ICD11: 2C60-2C6Z] | Approved | [1] | |||
| Structure |
|
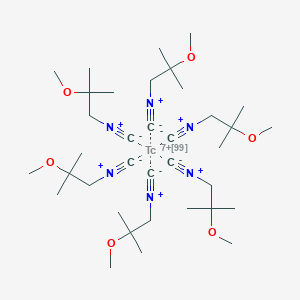 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C36H66N6O6Tc+7
|
|||||
| Canonical SMILES |
CC(C)(C[N+]#[C-])OC.CC(C)(C[N+]#[C-])OC.CC(C)(C[N+]#[C-])OC.CC(C)(C[N+]#[C-])OC.CC(C)(C[N+]#[C-])OC.CC(C)(C[N+]#[C-])OC.[Tc+7]
|
|||||
| InChI |
InChI=1S/6C6H11NO.Tc/c6*1-6(2,8-4)5-7-3;/h6*5H2,1-2,4H3;/q;;;;;;+7/i;;;;;;1+1
|
|||||
| InChIKey |
ZGDBEIODOXIKGQ-KTTJZPQESA-N
|
|||||
| CAS Number |
CAS 109581-73-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 777.9 | Topological Polar Surface Area | 81.5 | ||
| Heavy Atom Count | 49 | Rotatable Bond Count | 12 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 12 | |||
| PubChem CID | ||||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [2] | |
| References | ||||||
| 1 | 99mTc-sestamibi was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | 99mTc-sestamibi is a substrate for P-glycoprotein and the multidrug resistance-associated protein. Br J Cancer. 1998;77(3):353-8. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
