Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01385
|
|||||
| Drug Name |
Labetalol
|
|||||
| Synonyms |
Albetol; Ibidomide; Labetalolum; Labetolol; NORMOZIDE; AH 5158; AH-5158; Labetalol (INN); Labetalol [INN:BAN]; Labetalolum [INN-Latin]; Normodyne (TN); Sch-19927; Trandate (TN); 2-Hydroxy-5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)amino)ethyl)benzamide; 2-hydroxy-5-[1-hydroxy-2-(4-phenylbutan-2-ylamino)ethyl]benzamide; 2-hydroxy-5-{1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl}benzamide; 3-Carboxamido-4-hydroxy-alpha-((1-methyl-3-phenylpropylamino)methyl)benzyl alcohol; 5-(1-Hydroxy-2-(1-methyl-3-phenylpropylamino)ethyl)salicylamide
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Hypertension [ICD11: BA00] | Approved | [1] | |||
| Therapeutic Class |
Antihypertensive Agents
|
|||||
| Structure |
|
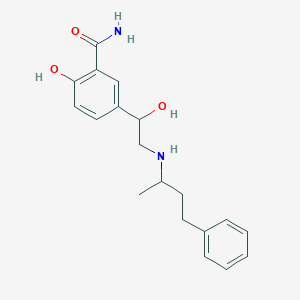 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C19H24N2O3
|
|||||
| Canonical SMILES |
CC(CCC1=CC=CC=C1)NCC(C2=CC(=C(C=C2)O)C(=O)N)O
|
|||||
| InChI |
InChI=1S/C19H24N2O3/c1-13(7-8-14-5-3-2-4-6-14)21-12-18(23)15-9-10-17(22)16(11-15)19(20)24/h2-6,9-11,13,18,21-23H,7-8,12H2,1H3,(H2,20,24)
|
|||||
| InChIKey |
SGUAFYQXFOLMHL-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 36894-69-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 328.4 | Topological Polar Surface Area | 95.6 | ||
| Heavy Atom Count | 24 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
3.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
9275
, 5142009
, 7979714
, 8152441
, 11335649
, 11360888
, 11364501
, 11367063
, 11369625
, 11372741
, 11373866
, 11377787
, 11461860
, 11466305
, 11467425
, 11484641
, 11486109
, 11488679
, 11491396
, 11491999
, 11495421
, 14777379
, 29222985
, 46505511
, 47216734
, 47736423
, 47885363
, 48184950
, 48259181
, 48259182
, 48259183
, 48259184
, 48416150
, 49698939
, 49832659
, 49876891
, 50105268
, 53790399
, 56413046
, 56464163
, 57322029
, 77168696
, 85209992
, 85788805
, 90341718
, 92716935
, 96024796
, 103165317
, 104304757
, 118314781
|
|||||
| ChEBI ID |
ChEBI:6343
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Labetalol was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Involvement of influx and efflux transport systems in gastrointestinal absorption of celiprolol. J Pharm Sci. 2009 Jul;98(7):2529-39. | |||||
| 3 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
