Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01398
|
|||||
| Drug Name |
Aprepitant
|
|||||
| Synonyms |
Aprepitant [USAN]; L 754030; MK 0869; Emend (TN); L-754030; MK-0869; ONO-7436; Aprepitant (JAN/USAN/INN); MK-869, L-754030, Emend, Aprepitant; 3-(((2R,3S)-3-(p-Fluorophenyl)-2-(((alphaR)-alpha-methyl-3,5-bis(trifluoromethyl)benzyl)oxy)morpholino)methyl)-Delta(2)-1,2,4-triazolin-5-one; 3-(((2R,3S)-3-(p-Fluorophenyl)-2-(((alphaR)-alpha-methyl-3,5-bis(trifluoromethyl)benzyl)oxy)morpholino)methyl)-delta(sup 2)-1,2,4-triazolin-5-one; 5-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)morpholin-4-yl]methyl]-1,2-dihydro-1,2,4-triazol-3-one; 5-[[(2S,3R)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)morpholin-4-yl]methyl]-1,2-dihydro-1,2,4-triazol-3-one; 5-{[(2R,3S)-2-{(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy}-3-(4-fluorophenyl)morpholin-4-yl]methyl}-2,4-dihydro-3H-1,2,4-triazol-3-one
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Nausea and vomiting [ICD11: MD90] | Approved | [1] | |||
| Therapeutic Class |
Antiemetics
|
|||||
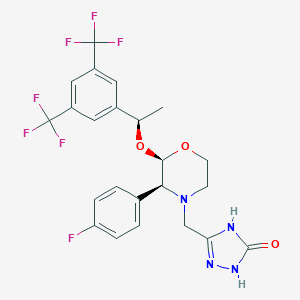
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C23H21F7N4O3
|
|||||
| Canonical SMILES |
CC(C1=CC(=CC(=C1)C(F)(F)F)C(F)(F)F)OC2C(N(CCO2)CC3=NNC(=O)N3)C4=CC=C(C=C4)F
|
|||||
| InChI |
InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1
|
|||||
| InChIKey |
ATALOFNDEOCMKK-OITMNORJSA-N
|
|||||
| CAS Number |
CAS 170729-80-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 534.4 | Topological Polar Surface Area | 75.2 | ||
| Heavy Atom Count | 37 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 12 | |||
| XLogP |
4.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
12015114
, 14787735
, 14812125
, 14861083
, 17194840
, 17397125
, 43529742
, 46505211
, 57371901
, 78987111
, 85688297
, 87325160
, 92309268
, 99004566
, 99436929
, 103537416
, 104093182
, 114787856
, 124757066
, 125163870
, 126591284
, 127455160
, 131407867
, 134340233
, 136920424
, 137218139
, 142087425
, 144115619
, 144241223
, 152034917
, 152108763
, 152258074
, 152344114
, 160646913
, 162011655
, 162037494
, 162180403
, 162802005
, 164831883
, 174527912
, 175268021
, 175611987
, 176484545
, 176484962
, 177749355
, 178100479
, 179150056
, 184816401
, 188899509
, 223704605
|
|||||
| ChEBI ID |
CHEBI:499361
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Aprepitant was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev. 2012 Jan;64(1):95-109. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
