Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01418
|
|||||
| Drug Name |
Acrivastine
|
|||||
| Synonyms |
Acrivastin; Acrivastina; Acrivastinum; Semprex; Acrivastina [Spanish]; Acrivastinum [Latin]; Benadryl allergy relief; BW 0270C; BW 825C; BW A825C; BW-825C; Acrivastine (USAN/INN); Acrivastine [USAN:INN:BAN]; E-(9CI); (2E)-3-{6-[(1E)-1-(4-methylphenyl)-3-pyrrolidin-1-ylprop-1-en-1-yl]pyridin-2-yl}prop-2-enoic acid; (E)-3-[6-[(E)-1-(4-methylphenyl)-3-pyrrolidin-1-ylprop-1-enyl]pyridin-2-yl]prop-2-enoic acid; (E)-6-((E)-3-(1-Pyrrolidinyl)-1-p-tolylpropenyl)-2-pyridineacrylic acid; (E)-6-((E)-3-(1-Pyrrolidinyl-1-p-tolylpropenyl)-2-pyridinacrylsaeure; (E,E)-3-[6-[1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl]-2-pyridinyl]-2-propenoic acid; 3-{6-[1-(4-methylphenyl)-3-(pyrrolidin-1-yl)prop-1-en-1-yl]pyridin-2-yl}prop-2-enoic acid
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Seasonal allergic rhinitis [ICD11: CA08.01] | Approved | [1] | |||
| Therapeutic Class |
Antiinflammatory Agents
|
|||||
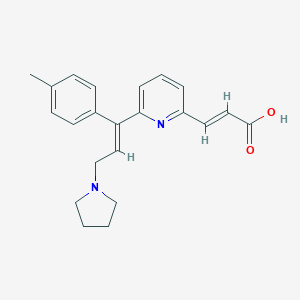
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C22H24N2O2
|
|||||
| Canonical SMILES |
CC1=CC=C(C=C1)C(=CCN2CCCC2)C3=CC=CC(=N3)C=CC(=O)O
|
|||||
| InChI |
InChI=1S/C22H24N2O2/c1-17-7-9-18(10-8-17)20(13-16-24-14-2-3-15-24)21-6-4-5-19(23-21)11-12-22(25)26/h4-13H,2-3,14-16H2,1H3,(H,25,26)/b12-11+,20-13+
|
|||||
| InChIKey |
PWACSDKDOHSSQD-IUTFFREVSA-N
|
|||||
| CAS Number |
CAS 87848-99-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 348.4 | Topological Polar Surface Area | 53.4 | ||
| Heavy Atom Count | 26 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
1.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
7978641
, 11039320
, 11528790
, 12012900
, 14827611
, 17396918
, 39317835
, 48415515
, 50478506
, 57359099
, 92719198
, 92729786
, 93166367
, 93309492
, 99299401
, 103374556
, 104178336
, 109614385
, 113863077
, 117544906
, 124893773
, 126628312
, 126651909
, 126670557
, 127327803
, 134338557
, 135260703
, 137100857
, 142971128
, 144206566
, 162180352
, 175268536
, 179151367
, 185967402
, 196106033
, 223447811
, 223683999
, 223704756
, 226396645
, 252344698
|
|||||
| ChEBI ID |
CHEBI:83168
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Acrivastine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. J Pharmacol Exp Ther. 2002 Dec;303(3):1029-37. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
