Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01419
|
|||||
| Drug Name |
Methysergide
|
|||||
| Synonyms |
Deseril; Desernil; Desernyl; Deseryl; Desril; Dimethylergometrin; Methylmethylergonovine; Methysergid; Methysergidum; Metisergide; Metisergido; Sansert; Methyllysergic acid butanolamide; Metisergide [DCIT]; UML 491; Deseril (TN); Methysergidum [INN-Latin]; Metisergido [INN-Spanish]; Sansert (TN); UML-491; Methysergide (USAN/INN); Methysergide [USAN:INN:BAN]; N-(alpha-(Hydroxymethyl)propyl)-1-methyl-dextro-lysergamide; N-(1-(Hydroxymethyl)propyl)-1-methyl-dextro-(+)-lysergamide; (+)-9,10-Didehydro-N-(1-(hydroxymethyl)propyl)-1,6-dimethylergoline-8beta-carboxamide; (+)-N-(1-(Hydroxymethyl)propyl)-1-methyl-D-lysergamide; (8beta)-N-[(1S)-1-(hydroxymethyl)propyl]-1,6-dimethyl-9,10-didehydroergoline-8-carboxamide; 1-Methyl-D-lysergic acid butanolamide; 1-Methyl-dextro-lysergic acid (+)-1-hydroxy-2-butylamide; 1-Methylmethylergonovine; 9,10-Didehydro-N-(1-(hydroxymethyl)propyl)-1,6-dimethylergoline-8-carboxamide
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Migraine [ICD11: 8A80] | Approved | [1] | |||
| Therapeutic Class |
Vasoconstrictor Agents
|
|||||
| Structure |
|
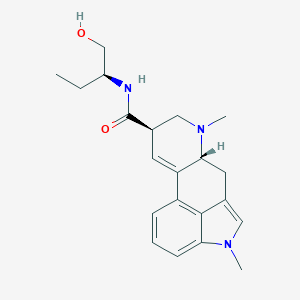 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C21H27N3O2
|
|||||
| Canonical SMILES |
CCC(CO)NC(=O)C1CN(C2CC3=CN(C4=CC=CC(=C34)C2=C1)C)C
|
|||||
| InChI |
InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1
|
|||||
| InChIKey |
KPJZHOPZRAFDTN-ZRGWGRIASA-N
|
|||||
| CAS Number |
CAS 361-37-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 353.5 | Topological Polar Surface Area | 57.5 | ||
| Heavy Atom Count | 26 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
2.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
9408
, 7849416
, 7979958
, 8156955
, 26751590
, 29228253
, 47365379
, 47515489
, 48334682
, 48416266
, 49965410
, 50104228
, 50428725
, 53790023
, 57325757
, 85787459
, 90340563
, 92309300
, 103292415
, 103940495
, 104321196
, 124750057
, 124886858
, 124886859
, 128415730
, 134337676
, 134973635
, 135650596
, 137002477
, 144204446
, 160963595
, 170464684
, 175266387
, 176484551
, 179236187
, 221673418
, 226426779
, 252614966
|
|||||
| ChEBI ID |
CHEBI:92629
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Methysergide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. J Pharmacol Exp Ther. 2002 Dec;303(3):1029-37. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
