Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01623
|
|||||
| Drug Name |
Eslicarbazepine acetate
|
|||||
| Synonyms |
Eslicarbazepine acetate; BIA 2-093; Aptiom; Zebinix; Stedesa; Exalief; Eslicarbazepine (acetate); UNII-BEA68ZVB2K; BIA-2-093; (S)-5-Carbamoyl-10,11-dihydro-5H-dibenzo[b,f]azepin-10-yl acetate; Sep 0002093; BIA 2093; BEA68ZVB2K; SEP-0002093; CHEMBL87992; Eslicarbazepine acetate [USAN]; CHEBI:87016; exelief; (S)-(-)-10-Acetoxy-10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide; [(5S)-11-carbamoyl-5,6-dihydrobenzo[b][1]benzazepin-5-yl] acetate; Eslicarbazepine acetate (USAN)
|
|||||
| Indication | Partial seizures [ICD11: 8A68.0] | Approved | [1] | |||
| Structure |
|
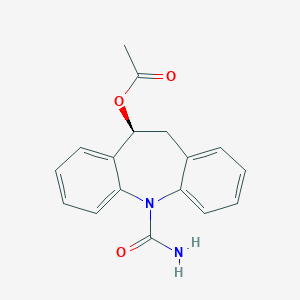 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C17H16N2O3
|
|||||
| Canonical SMILES |
CC(=O)OC1CC2=CC=CC=C2N(C3=CC=CC=C13)C(=O)N
|
|||||
| InChI |
InChI=1S/C17H16N2O3/c1-11(20)22-16-10-12-6-2-4-8-14(12)19(17(18)21)15-9-5-3-7-13(15)16/h2-9,16H,10H2,1H3,(H2,18,21)/t16-/m0/s1
|
|||||
| InChIKey |
QIALRBLEEWJACW-INIZCTEOSA-N
|
|||||
| CAS Number |
CAS 236395-14-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 296.32 | Topological Polar Surface Area | 72.6 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
2
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:87016
|
|||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Eslicarbazepine acetate was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | In vitro transport profile of carbamazepine, oxcarbazepine, eslicarbazepine acetate, and their active metabolites by human P-glycoprotein. Epilepsia. 2011 Oct;52(10):1894-904. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
