Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01644
|
|||||
| Drug Name |
Bouvardin
|
|||||
| Synonyms |
Bouvardin; MLS002703036; NSC259968; NSC 259968; NSC-259968; AC1L8NRS; Neuro_000123; SCHEMBL8739404; SMR001566844; From fraction F049 of Bouvardia ternifolia; Cyclic(D-alanyl-L-alanyl-N, cyclic(54.fwdarw.63)-ether, (S)-; 17,24-dihydroxy-10-(4-methoxybenzyl)-4,7,9,13,15,29-hexamethyl-22-oxa-3,6,9,12,15,29-hexaazatetracyclo[14.12.2.2~18,21~.1~23,27~]tritriaconta-18,20,23(31),24,26,32-hexaene-2,5,8,11,14,30-hexone
|
|||||
| Indication | Cancer [ICD11: 2A00-2F9Z] | Preclinical | [1] | |||
| Structure |
|
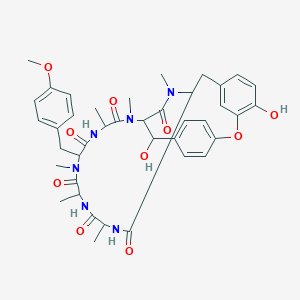 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C40H48N6O10
|
|||||
| Canonical SMILES |
CC1C(=O)NC(C(=O)N(C(C(=O)NC(C(=O)N(C2C(C3=CC=C(C=C3)OC4=C(C=CC(=C4)CC(C(=O)N1)N(C2=O)C)O)O)C)C)CC5=CC=C(C=C5)OC)C)C
|
|||||
| InChI |
InChI=1S/C40H48N6O10/c1-21-35(49)42-22(2)38(52)44(4)29(18-24-8-13-27(55-7)14-9-24)37(51)43-23(3)39(53)46(6)33-34(48)26-11-15-28(16-12-26)56-32-20-25(10-17-31(32)47)19-30(36(50)41-21)45(5)40(33)54/h8-17,20-23,29-30,33-34,47-48H,18-19H2,1-7H3,(H,41,50)(H,42,49)(H,43,51)
|
|||||
| InChIKey |
TWOWSRSKGJSZHZ-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 64755-14-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 772.8 | Topological Polar Surface Area | 207 | ||
| Heavy Atom Count | 56 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 5 | Hydrogen Bond Acceptor Count | 10 | |||
| XLogP |
1.9
|
|||||
| PubChem CID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Total Synthesis of Bouvardin, O-Methylbouvardin, and O-Methyl-N9-desmethylbouvardin | |||||
| 2 | Fingerprint-based in silico models for the prediction of P-glycoprotein substrates and inhibitors. Bioorg Med Chem. 2012 Sep 15;20(18):5388-95. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
