Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01679
|
|||||
| Drug Name |
Lovastatin acid
|
|||||
| Synonyms |
Lovastatin acid; Mevinolinic acid; Monacolinic K acid; MSD 803 acid; MSD 803 free acid; UNII-5CLV35Y90C; MK 819; L-154819; L 154819; 5CLV35Y90C; CHEBI:82985; (3R,5R)-7-((1R,2R,6S,8R,8AS)-2,6-DIMETHYL-8-{[(2R)-2-METHYLBUTANOYL]OXY}-1,2,6,7,8,8A-HEXAHYDRONAPHTHALEN-1-YL)-3,5-DIHYDROXYHEPTANOIC ACID; (3R,5R)-7-[(1S,2S,6R,8S,8aR)-2,6-dimethyl-8-{[(2S)-2-methylbutanoyl]oxy}-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-dihydroxyheptanoic acid
|
|||||
| Indication | Hypercholesterolemia [ICD11: 5C80.0] | Approved | [1] | |||
| Structure |
|
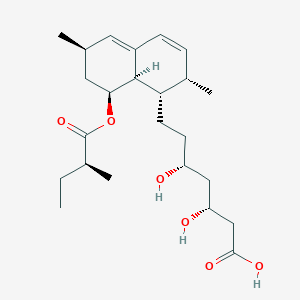 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C24H38O6
|
|||||
| Canonical SMILES |
CCC(C)C(=O)OC1CC(C=C2C1C(C(C=C2)C)CCC(CC(CC(=O)O)O)O)C
|
|||||
| InChI |
InChI=1S/C24H38O6/c1-5-15(3)24(29)30-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-18(25)12-19(26)13-22(27)28/h6-7,10,14-16,18-21,23,25-26H,5,8-9,11-13H2,1-4H3,(H,27,28)/t14-,15-,16-,18+,19+,20-,21-,23-/m0/s1
|
|||||
| InChIKey |
QLJODMDSTUBWDW-BXMDZJJMSA-N
|
|||||
| CAS Number |
CAS 75225-51-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 422.6 | Topological Polar Surface Area | 104 | ||
| Heavy Atom Count | 30 | Rotatable Bond Count | 11 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
3.5
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:82985
|
|||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Lovastatin acid was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
