Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01689
|
|||||
| Drug Name |
Tenofovir disoproxil
|
|||||
| Synonyms |
Tenofovir disoproxil; PMPA prodrug; Bis(POC)PMPA; Tenofovir (Disoproxil); UNII-F4YU4LON7I; 9-((R)-2-((Bis(((isopropoxycarbonyl)oxy)methoxy)phosphinyl)methoxy)propyl)adenine; F4YU4LON7I; CHEBI:63717; tenofovir bis(isopropyloxycarbonyloxymethyl) ester; Tenofovir Disoproxil Fumarate; [[(1R)-2-(6-aminopurin-9-yl)-1-methyl-ethoxy]methyl-(isopropoxycarbonyloxymethoxy)phosphoryl]oxymethyl isopropyl carbonate
|
|||||
| Indication | Chronic hepatitis B infection [ICD11: 1E51.0] | Phase 4 | [1] | |||
| Structure |
|
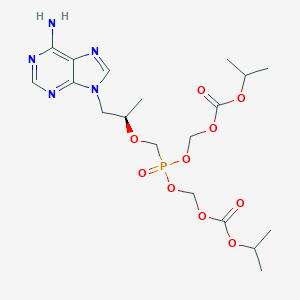 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C19H30N5O10P
|
|||||
| Canonical SMILES |
CC(C)OC(=O)OCOP(=O)(COC(C)CN1C=NC2=C(N=CN=C21)N)OCOC(=O)OC(C)C
|
|||||
| InChI |
InChI=1S/C19H30N5O10P/c1-12(2)33-18(25)28-9-31-35(27,32-10-29-19(26)34-13(3)4)11-30-14(5)6-24-8-23-15-16(20)21-7-22-17(15)24/h7-8,12-14H,6,9-11H2,1-5H3,(H2,20,21,22)/t14-/m1/s1
|
|||||
| InChIKey |
JFVZFKDSXNQEJW-CQSZACIVSA-N
|
|||||
| CAS Number |
CAS 201341-05-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 519.4 | Topological Polar Surface Area | 185 | ||
| Heavy Atom Count | 35 | Rotatable Bond Count | 17 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 14 | |||
| XLogP |
1.6
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:63717
|
|||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | ClinicalTrials.gov (NCT03485534) Evaluate the Efficacy and Safety to Tenofovir Disoproxil in Chronic Hepatitis B Patients | |||||
| 2 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
