Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01726
|
|||||
| Drug Name |
Imipenem
|
|||||
| Synonyms |
imipenem; 64221-86-9; Imipemide; N-Formimidoylthienamycin; Imipenem anhydrous; Tienamycin; Imipenemum; Imipenen; N-formimidoyl thienamycin; Imipenem hydrate; MK 0787; CHEBI:471744; 74431-23-5; Imipenem, N-Formimidoyl thienamycin; (5R,6S)-3-[2-(aminomethylideneamino)ethylsulfanyl]-6-[(1R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; Imipenem (INN); Imipenem [INN]; Imipenemum [Latin]; MK-0787; (5R,6S)-6-((R)-1-Hydroxyethyl)-3-(2-(iminomethylamino)ethylthio)-7-oxo-1-azabicyclo(3.2.0)hept-2-ene-2-carbonsaeure; (5R,6S)-3-((2-(Formimidoylamino)ethyl)thio)-6-((R)-1-hydroxyethyl)-7-oxo-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic acid; CHEMBL148; UNII-Q20IM7HE75; Q20IM7HE75; DTXSID2023143; Imipenem, Anhydrous; EINECS 264-734-5; MK-787; (5R,6S)-3-(2-Formimidoylamino-ethylsulfanyl)-6-((R)-1-hydroxy-ethyl)-7-oxo-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylic acid; Imipenem-1-wasser; DTXCID903143; C12H17N3O4S; Anhydrous Imipenem; C12H17N3O4S.H2O; Imipenem [USAN:INN:BAN:JAN]; (5R,6S)-6-[(1R)-1-hydroxyethyl]-3-({2-[(iminomethyl)amino]ethyl}thio)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (5R,6S)-6-[(1R)-1-hydroxyethyl]-3-[(2-methanimidamidoethyl)sulfanyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; CAS-64221-86-9; MK0787; SR-05000000294; C12-H17-N3-O4-S.H2-O; imipen; NCGC00016928-01; Primaxin (TN); (5R,6S)-6-[(1R)-1-hydroxyethyl]-3-(2-methanimidamidoethylsulfanyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; 1-Azabicyclo(3.2.0)hept-2-ene-2-carboxylic acid, 6-((1R)-1-hydroxyethyl)-3-((2-((iminomethyl)amino)ethyl)thio)-7-oxo-, (5R,6S)-; 1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid, 6-[(1R)-1-hydroxyethyl]-3-[[2-[(iminomethyl)amino]ethyl]thio]-7-oxo-, (5R,6S)-; Prestwick_844; IMIPENEM [MI]; IMIPENEM ANHYDRATE; N-formimidoylthien-amycin; (5R,6S)-3-((2-(Formimidoylamino)ethyl)thio)-6-((R)-1-hydroxyethyl)-7-oxo-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic acid monohydrate; Prestwick0_000519; Prestwick1_000519; Prestwick2_000519; Prestwick3_000519; D0H3TD; Epitope ID:120384; IMIPENEM [WHO-DD]; BSPBio_000477; BIDD:GT0686; SPBio_002398; BPBio1_000525; SCHEMBL1649260; SCHEMBL8781920; GTPL10821; HY-B1369A; Primaxin (imipenem + cilastatin); ZSKVGTPCRGIANV-ZXFLCMHBSA-N; HMS1569H19; HMS2090A15; HMS2096H19; HMS3260H20; HMS3713H19; Pharmakon1600-01506001; 1-Azabicyclo(3.2.0)hept-2-ene-2-carboxylic acid, 6-(1-hydroxyethyl)-3-((2-((iminomethyl)amino)ethyl)thio)-7-oxo-, (5R-(5-alpha,6-alpha(R*)))-; BCP13012; Tox21_110689; Tox21_500279; BDBM50049708; BDBM50213266; NSC717864; NSC759901; AKOS016010844; Tox21_110689_1; CCG-220519; CCG-221583; DB01598; LP00279; NSC-717864; SDCCGSBI-0633697.P001; NCGC00167958-01; NCGC00167958-02; NCGC00167958-03; NCGC00167958-05; NCGC00167958-09; NCGC00260964-01; 1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid,6-[(1R)-1-hydroxyethyl]-3-[[2-[(iminomethyl)amino]ethyl]thio]-7-oxo-,(5R,6S)-; AS-75130; CS-0077844; C06665; D04515; D96091; AB01563339_01; Recarbrio (imipenem + cilastatin + relebactam).; EN300-19766283; Q425152; SR-05000000294-2; SR-05000000294-5; Thienamycin p-nitrobenzylester hydrochloride(N-methylpyrrolidinonesolvate); (5R,6S)-3-((2-formimidamidoethyl)thio)-6-((R)-1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (5R,6S)-3-({2-[(E)-(aminomethylidene)amino]ethyl}sulfanyl)-6-[(1R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (5R,6S)-3-(2-formimidamidoethylthio)-6-((R)-1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; [5R-[5.alpha.,6.alpha.(R*)]]-6-(1-Hydroxyethyl)-3-[[2- [(iminomethyl)amino]ethyl]thio]-7-oxo-1-azabicyclo[3.2.0]hept-2- ene-2-carboxylic acid monohydrate; [5R-[5.alpha.,6.alpha.(R*)]]-6-(1-Hydroxyethyl)-3-[[2-[(iminomethyl)amino]ethyl]thio]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monohydrate; 1-AZABICYCLO(3.2.0)HEPT-2-ENE-2-CARBOXYLIC ACID, 6-(1-HYDROXYETHYL)-3-((2-((IMINOMETHYL)AMINO)ETHYL)THIO)-7-OXO-, (5R-(5.ALPHA.,6.ALPHA.(R*)))-
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Bacterial infections [ICD11: 1A00-1H0Z] | Approved | [1] | |||
| Structure |
|
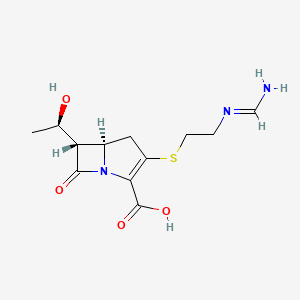 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C12H17N3O4S
|
|||||
| Canonical SMILES |
CC(C1C2CC(=C(N2C1=O)C(=O)O)SCCN=CN)O
|
|||||
| InChI |
InChI=1S/C12H17N3O4S/c1-6(16)9-7-4-8(20-3-2-14-5-13)10(12(18)19)15(7)11(9)17/h5-7,9,16H,2-4H2,1H3,(H2,13,14)(H,18,19)/t6-,7-,9-/m1/s1
|
|||||
| InChIKey |
ZSKVGTPCRGIANV-ZXFLCMHBSA-N
|
|||||
| CAS Number |
CAS 64221-86-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 299.35 | Topological Polar Surface Area | 142 | ||
| Heavy Atom Count | 20 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
-0.7
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:471744
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [2] | |
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [2] | ||
| References | ||||||
| 1 | Imipenem was approved by FDA. The official website of the U.S. Food and Drug Administration. (2023) | |||||
| 2 | Organic anion transporters also mediate the drug-drug interaction between imipenem and cilastatin. Asian J Pharm Sci. 2020 Mar;15(2):252-263. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
