Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00030
|
|||||
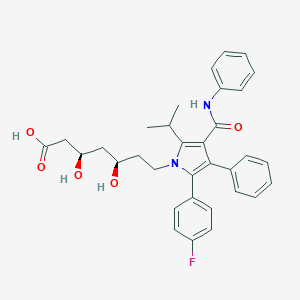
| Drug Name |
Atorvastatin
|
|||||
| Synonyms |
(3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid; (3R,5R)-7-[3-(anilinocarbonyl)-5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid; (R-(R*,R*))-2-(4-Fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-((phenylamino)carbonyl)-1H-pyrrole-1-heptanoic acid; (betaR,deltaR)-2-(p-Fluorophenyl)-beta,delta-dihydroxy-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrole-1-heptanoic acid; 7-[2-(4-FLUORO-PHENYL)-5-ISOPROPYL-3-PHENYL-4-PHENYLCARBAMOYL-PYRROL-1-YL]-3,5-DIHYDROXY-HEPTANOIC ACID; Atogal; Atorlip; Atorpic; Atorvastatin (INN); Atorvastatin [INN:BAN]; Atorvastatina; Atorvastatine; Atorvastatinium; Atrovastin; Cardyl; Faboxim; Lipitor (TN); Lipitor(TM); Lipotropic; Lipovastatinklonal; Liprimar; Lowden; Sincol; Sortis; Sortis (TN); Sotis; Torvacard; Torvast; Totalip; Tozalip; Tulip; Vastina; Xanator; Xarator; Xavator; Zurinel
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Heart attack [ICD11: BA41.Z] | Approved | [1] | |||
| Dyslipidaemias [ICD11: 5C8Z] | Approved | [1] | ||||
| Hyperlipidaemia [ICD11: 5C8Z] | Approved | [1] | ||||
| Therapeutic Class |
Anticholesteremic Agents
|
|||||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C33H35FN2O5
|
|||||
| Canonical SMILES |
CC(C)C1=C(C(=C(N1CCC(CC(CC(=O)O)O)O)C2=CC=C(C=C2)F)C3=CC=CC=C3)C(=O)NC4=CC=CC=C4
|
|||||
| InChI |
InChI=1S/C33H35FN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40)/t26-,27-/m1/s1
|
|||||
| InChIKey |
XUKUURHRXDUEBC-KAYWLYCHSA-N
|
|||||
| CAS Number |
CAS 134523-00-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 558.6 | Topological Polar Surface Area | 112 | ||
| Heavy Atom Count | 41 | Rotatable Bond Count | 12 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103554720
, 104178840
, 104321734
, 104829550
, 117367061
, 124892211
, 126525305
, 126624171
, 126658151
, 126682126
, 127315782
, 127315783
, 127315784
, 127315785
, 127315786
, 127315787
, 127315788
, 127315789
, 127315790
, 127315791
, 127315792
, 127315793
, 14910832
, 26684326
, 26697359
, 29215408
, 43118161
, 49845979
, 50037926
, 51091801
, 53787715
, 56311281
, 56311722
, 56311943
, 56311969
, 56312802
, 56313042
, 56313334
, 56313578
, 56314463
, 57314133
, 75432172
, 7884979
, 7978735
, 8187078
, 822166
, 85856289
, 9052
, 92714388
, 93166494
|
|||||
| ChEBI ID |
ChEBI:39548
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MCT1 | Transporter Info | Monocarboxylate transporter 1 | Substrate | [2] | |
| MCT2 | Transporter Info | Monocarboxylate transporter 2 | Substrate | [3] | ||
| OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [4] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [5] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [6] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [7] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [8] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OATP1B1 | Transporter Info | Km = 0.77 microM | Human embryonic kidney cells (HEK293)-OATP1B1 | [9] | |
| OATP1B1 | Transporter Info | Km = 12.4 microM | Human embryonic kidney cells (HEK293)-OATP1B1 | [10] | ||
| OATP1B1 | Transporter Info | Km = 18.9 microM | Human embryonic kidney cells (HEK293)-OATP1B1 | [11] | ||
| OATP1B3 | Transporter Info | Km = 0.73 microM | Human embryonic kidney cells (HEK293)-OATP1B3 | [9] | ||
| OATP2B1 | Transporter Info | Km = 2.84 microM | Human embryonic kidney cells (HEK293)-OATP2B1 | [9] | ||
| OATP2B1 | Transporter Info | Km = 0.2 microM | Madin-Darby canine kidney cells (MDCKII)-OATP2B1 | [12] | ||
| References | ||||||
| 1 | Ramipril was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol. 2007 Jul;92(4):603-19. | |||||
| 3 | Monocarboxylate Transporters in Drug Disposition: Role in the Toxicokinetics and Toxicodynamics of the Drug of Abuse GHB. | |||||
| 4 | Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1A2 and 2B1. Biochem Pharmacol. 2010 Dec 1;80(11):1746-53. | |||||
| 5 | Rifampicin alters atorvastatin plasma concentration on the basis of SLCO1B1 521T>C polymorphism. Clin Chim Acta. 2009 Jul;405(1-2):49-52. | |||||
| 6 | FDA Drug Development and Drug Interactions | |||||
| 7 | Human platelets express organic anion-transporting peptide 2B1, an uptake transporter for atorvastatin. Drug Metab Dispos. 2009 May;37(5):1129-37. | |||||
| 8 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | |||||
| 9 | Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug-drug interactions. J Med Chem. 2012 May 24;55(10):4740-63. | |||||
| 10 | Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics. 2005 Jul;15(7):513-22. | |||||
| 11 | effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007 Feb;81(2):194-204. | |||||
| 12 | Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther. 2006 Dec;80(6):607-20. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
